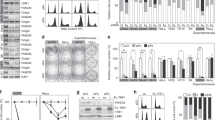
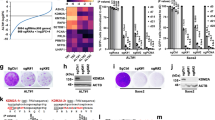
Abstract
Most cancer cells activate telomerase to elongate telomeres and achieve unlimited replicative potential. Some cancer cells cannot activate telomerase and use telomere homologous recombination (HR) to elongate telomeres, a mechanism termed alternative lengthening of telomeres (ALT). A hallmark of ALT cells is the recruitment of telomeres to PML bodies (termed APBs). Here, we show that the SMC5/6 complex localizes to APBs in ALT cells and is required for targeting telomeres to APBs. The MMS21 SUMO ligase of the SMC5/6 complex SUMOylates multiple telomere-binding proteins, including TRF1 and TRF2. Inhibition of TRF1 or TRF2 SUMOylation prevents APB formation. Depletion of SMC5/6 subunits by RNA interference inhibits telomere HR, causing telomere shortening and senescence in ALT cells. Thus, the SMC5/6 complex facilitates telomere HR and elongation in ALT cells by promoting APB formation through SUMOylation of telomere-binding proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blackburn, E.H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
Harley, C.B., Futcher, A.B. & Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Bodnar, A.G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Smogorzewska, A. & de Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73, 177–208 (2004).
Cong, Y.S., Wright, W.E. & Shay, J.W. Human telomerase and its regulation. Microbiol. Mol. Biol. Rev. 66, 407–425 (2002).
Shay, J.W. & Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 33, 787–791 (1997).
Bryan, T.M., Englezou, A., Dalla-Pozza, L., Dunham, M.A. & Reddel, R.R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 3, 1271–1274 (1997).
Henson, J.D. et al. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin. Cancer Res. 11, 217–225 (2005).
Bryan, T.M., Englezou, A., Gupta, J., Bacchetti, S. & Reddel, R.R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 14, 4240–4248 (1995).
Muntoni, A. & Reddel, R.R. The first molecular details of ALT in human tumor cells. Hum. Mol. Genet. 14, R191–R196 (2005).
Henson, J.D., Neumann, A.A., Yeager, T.R. & Reddel, R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610 (2002).
Lundblad, V. & Blackburn, E.H. An alternative pathway for yeast telomere maintenance rescues est1-senescence. Cell 73, 347–360 (1993).
Dunham, M.A., Neumann, A.A., Fasching, C.L. & Reddel, R.R. Telomere maintenance by recombination in human cells. Nat. Genet. 26, 447–450 (2000).
Londono-Vallejo, J.A., Der-Sarkissian, H., Cazes, L., Bacchetti, S. & Reddel, R.R. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 64, 2324–2327 (2004).
Rogan, E.M. et al. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol. Cell. Biol. 15, 4745–4753 (1995).
Yeager, T.R. et al. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 59, 4175–4179 (1999).
Perrem, K., Colgin, L.M., Neumann, A.A., Yeager, T.R. & Reddel, R.R. Coexistence of alternative lengthening of telomeres and telomerase in hTERT-transfected GM847 cells. Mol. Cell. Biol. 21, 3862–3875 (2001).
Grobelny, J.V., Godwin, A.K. & Broccoli, D. ALT-associated PML bodies are present in viable cells and are enriched in cells in the G(2)/M phase of the cell cycle. J. Cell Sci. 113, 4577–4585 (2000).
Johnson, F.B. et al. The Saccharomyces cerevisiae WRN homolog Sgs1p participates in telomere maintenance in cells lacking telomerase. EMBO J. 20, 905–913 (2001).
Nabetani, A., Yokoyama, O. & Ishikawa, F. Localization of hRad9, hHus1, hRad1, and hRad17 and caffeine-sensitive DNA replication at the alternative lengthening of telomeres-associated promyelocytic leukemia body. J. Biol. Chem. 279, 25849–25857 (2004).
Yankiwski, V., Marciniak, R.A., Guarente, L. & Neff, N.F. Nuclear structure in normal and Bloom syndrome cells. Proc. Natl. Acad. Sci. USA 97, 5214–5219 (2000).
Zhu, X.D., Kuster, B., Mann, M., Petrini, J.H. & de Lange, T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 25, 347–352 (2000).
Wu, G., Jiang, X., Lee, W.H. & Chen, P.L. Assembly of functional ALT-associated promyelocytic leukemia bodies requires Nijmegen Breakage Syndrome 1. Cancer Res. 63, 2589–2595 (2003).
Jiang, W.Q. et al. Suppression of alternative lengthening of telomeres by Sp100-mediated sequestration of the MRE11/RAD50/NBS1 complex. Mol. Cell. Biol. 25, 2708–2721 (2005).
Hirano, T. SMC proteins and chromosome mechanics: from bacteria to humans. Phil. Trans. R. Soc. Lond. B 360, 507–514 (2005).
Koshland, D.E. & Guacci, V. Sister chromatid cohesion: the beginning of a long and beautiful relationship. Curr. Opin. Cell Biol. 12, 297–301 (2000).
Swedlow, J.R. & Hirano, T. The making of the mitotic chromosome: modern insights into classical questions. Mol. Cell 11, 557–569 (2003).
Lehmann, A.R. The role of SMC proteins in the responses to DNA damage. DNA Repair (Amst.) 4, 309–314 (2005).
Lehmann, A.R. et al. The rad18 gene of Schizosaccharomyces pombe defines a new subgroup of the SMC superfamily involved in DNA repair. Mol. Cell. Biol. 15, 7067–7080 (1995).
Verkade, H.M., Bugg, S.J., Lindsay, H.D., Carr, A.M. & O'Connell, M.J. Rad18 is required for DNA repair and checkpoint responses in fission yeast. Mol. Biol. Cell 10, 2905–2918 (1999).
Torres-Rosell, J. et al. SMC5 and SMC6 genes are required for the segregation of repetitive chromosome regions. Nat. Cell Biol. 7, 412–419 (2005).
Lee, K.M. & O'Connell, M.J. A new SUMO ligase in the DNA damage response. DNA Repair (Amst.) 5, 138–141 (2006).
Muller, S., Hoege, C., Pyrowolakis, G. & Jentsch, S. SUMO, ubiquitin's mysterious cousin. Nat. Rev. Mol. Cell Biol. 2, 202–210 (2001).
Potts, P.R. & Yu, H. Human MMS21/NSE2 is a SUMO ligase required for DNA repair. Mol. Cell. Biol. 25, 7021–7032 (2005).
Zhao, X. & Blobel, G.A. SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc. Natl. Acad. Sci. USA 102, 4777–4782 (2005).
Potts, P.R., Porteus, M.H. & Yu, H. Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. EMBO J. 25, 3377–3388 (2006).
De Piccoli, G. et al. Smc5-Smc6 mediate DNA double-strand-break repair by promoting sister-chromatid recombination. Nat. Cell Biol. 8, 1032–1034 (2006).
Lindroos, H.B. et al. Chromosomal association of the Smc5/6 complex reveals that it functions in differently regulated pathways. Mol. Cell 22, 755–767 (2006).
Onoda, F. et al. SMC6 is required for MMS-induced interchromosomal and sister chromatid recombinations in Saccharomyces cerevisiae. DNA Repair (Amst.) 3, 429–439 (2004).
Wright, W.E., Pereira-Smith, O.M. & Shay, J.W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 9, 3088–3092 (1989).
Laud, P.R. et al. Elevated telomere-telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 19, 2560–2570 (2005).
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 (2005).
Gocke, C.B., Yu, H. & Kang, J. Systematic identification and analysis of mammalian small ubiquitin-like modifier substrates. J. Biol. Chem. 280, 5004–5012 (2005).
Lin, D.Y. et al. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell 24, 341–354 (2006).
Rodriguez, M.S., Dargemont, C. & Hay, R.T. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J. Biol. Chem. 276, 12654–12659 (2001).
Xhemalce, B. et al. Role of SUMO in the dynamics of telomere maintenance in fission yeast. Proc. Natl. Acad. Sci. USA 104, 893–898 (2007).
Maul, G.G., Negorev, D., Bell, P. & Ishov, A.M. Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 129, 278–287 (2000).
Borden, K.L. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol. Cell. Biol. 22, 5259–5269 (2002).
Wu, G., Lee, W.H. & Chen, P.L. NBS1 and TRF1 colocalize at promyelocytic leukemia bodies during late S/G2 phases in immortalized telomerase-negative cells. Implication of NBS1 in alternative lengthening of telomeres. J. Biol. Chem. 275, 30618–30622 (2000).
Seeler, J.S. & Dejean, A. SUMO: of branched proteins and nuclear bodies. Oncogene 20, 7243–7249 (2001).
Ishov, A.M. et al. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147, 221–234 (1999).
Ampatzidou, E., Irmisch, A., O'Connell, M.J. & Murray, J.M. Smc5/6 is required for repair at collapsed replication forks. Mol. Cell. Biol. 26, 9387–9401 (2006).
Branzei, D. et al. Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell 127, 509–522 (2006).
Miyabe, I., Morishita, T., Hishida, T., Yonei, S. & Shinagawa, H. Rhp51-dependent recombination intermediates that do not generate checkpoint signal are accumulated in Schizosaccharomyces pombe rad60 and smc5/6 mutants after release from replication arrest. Mol. Cell. Biol. 26, 343–353 (2006).
Wang, R.C., Smogorzewska, A. & de Lange, T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119, 355–368 (2004).
Acknowledgements
We thank W. Wright (University of Texas Southwestern Medical Center) for the SUSM1, SW26 and SW39 cell lines, D. Chen (University of Texas Southwestern Medical Center) for complementary DNAs encoding the telomere-binding proteins, and S. Chang (M.D. Anderson Cancer Center) for G5 Terc−/− Wrn−/− Rasv12 MEF ALT cells. We also thank members of our laboratory and the laboratories of D. Chen and W. Wright for helpful discussions. This work is supported by grants from the W.M. Keck Foundation (to H.Y.) and the Welch Foundation (to H.Y.), and by a US National Institutes of Health Pharmacological Sciences Training Grant (to P.R.P.).
Author information
Authors and Affiliations
Contributions
P.R.P. and H.Y. designed the research. P.R.P. performed the experiments and analyzed the results. P.R.P. and H.Y. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
SMC5–SMC6 localizes to PML bodies in telomerase–positive HeLa cells, but does in ALT cells (PDF 82 kb)
Supplementary Fig. 2
SMC5–SMC6 localizes to PML bodies in SW26 ALT but not in SW39 telomerase-positive cells (PDF 53 kb)
Supplementary Fig. 3
Cohesin complex does not localize to PML bodies (PDF 77 kb)
Supplementary Fig. 4
MMS21 sumoylates components of the shelterin complex (PDF 90 kb)
Supplementary Fig. 5
TRF2 sumoylation is required for TRF2 recruitment to PML bodies (PDF 90 kb)
Supplementary Fig. 6
Inhibition of the SMC5–SMC6 complex results in telomeric shortening (PDF 104 kb)
Supplementary Table 1
Knockdown of the SMC5–SMC6 complex in ALT cells results in telomere abnormalities (PDF 7 kb)
Rights and permissions
About this article
Cite this article
Potts, P., Yu, H. The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat Struct Mol Biol 14, 581–590 (2007). https://doi.org/10.1038/nsmb1259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1259
This article is cited by
-
The interactions between PML nuclear bodies and small and medium size DNA viruses
Virology Journal (2023)
-
The multi-functional Smc5/6 complex in genome protection and disease
Nature Structural & Molecular Biology (2023)
-
Histone demethylase KDM2A is a selective vulnerability of cancers relying on alternative telomere maintenance
Nature Communications (2023)
-
Smc5/6 silences episomal transcription by a three-step function
Nature Structural & Molecular Biology (2022)
-
Targeting telomeres: advances in telomere maintenance mechanism-specific cancer therapies
Nature Reviews Cancer (2022)