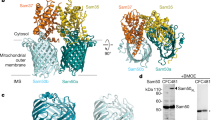
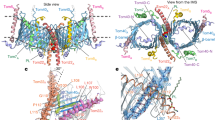
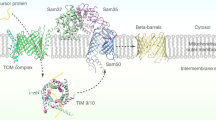
Abstract
The general preprotein translocase of the outer mitochondrial membrane (TOM complex) transports virtually all mitochondrial precursor proteins, but cannot assemble outer-membrane precursors into functional complexes. A recently discovered sorting and assembly machinery (SAM complex) is essential for integration and assembly of outer-membrane proteins, revealing unexpected connections to mitochondrial evolution and morphology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sickmann, A. et al. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA 100, 13207–13212 (2003).
Taylor, S.W. et al. Characterization of the human heart mitochondrial proteome. Nat. Biotechnol. 21, 281–286 (2003).
Lill, R. & Kispal, G. Maturation of cellular Fe-S proteins: an essential function of mitochondria. Trends Biochem. Sci. 25, 352–356 (2000).
Newmeyer, D.D. & Ferguson-Miller, S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112, 481–490 (2003).
Herrmann, J.M. & Neupert, W. Protein transport into mitochondria. Curr. Opin. Microbiol. 3, 210–214 (2000).
Jensen, R.E. & Dunn, C.D. Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim. Biophys. Acta 1592, 25–34 (2002).
Endo, T., Yamamoto, H. & Esaki, M. Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J. Cell Sci. 116, 3259–3267 (2003).
Koehler, C.M. New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20, 309–335 (2004).
Wiedemann, N., Frazier, A.E. & Pfanner, N. The protein import machinery of mitochondria. J. Biol. Chem. 279, 14473–14476 (2004).
Glick, B.S. et al. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell 69, 809–822 (1992).
Stuart, R.A. Insertion of proteins into the inner membrane of mitochondria: the role of the Oxa1 complex. Biochim. Biophys. Acta 1592, 79–87 (2002).
Pfanner, N., Tropschug, M. & Neupert, W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell 49, 815–823 (1987).
Wiedemann, N., Pfanner, N. & Ryan, M.T. The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria. EMBO J. 20, 951–960 (2001).
Vial, S. et al. Assembly of Tim9 and Tim10 into a functional chaperone. J. Biol. Chem. 277, 36100–36108 (2002).
Rehling, P. et al. Protein insertion into the mitochondrial inner membrane by a twin-pore translocase. Science 299, 1747–1751 (2003).
Gabriel, K., Buchanan, S.K. & Lithgow, T. The alpha and the beta: protein translocation across mitochondrial and plastid outer membranes. Trends Biochem. Sci. 26, 36–40 (2001).
Matouschek, A. & Glick, B.S. Barreling through the outer membrane. Nat. Struct. Biol. 8, 284–286 (2001).
Johnson, A.E. & Jensen, R.E. Barreling through the membrane. Nat. Struct. Mol. Biol. 11, 113–114 (2004).
Keil, P. et al. Biogenesis of the mitochondrial receptor complex: two receptors are required for binding of MOM38 to the outer membrane surface. J. Biol. Chem. 268, 19177–19180 (1993).
Krimmer, T. et al. Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the TOM complex. J. Cell Biol. 152, 289–300 (2001).
Rapaport, D. et al. Structural requirements of Tom40 for assembly into preexisting TOM complexes of mitochondria. Mol. Biol. Cell 12, 1189–1198 (2001).
Rapaport, D. Biogenesis of the mitochondrial TOM complex. Trends Biochem. Sci. 27, 191–197 (2002).
Künkele, K.-P. et al. The preprotein translocation channel of the outer membrane of mitochondria. Cell 93, 1009–1019 (1998).
van Wilpe, S. et al. Tom22 is a multifunctional organizer of the mitochondrial pre-protein translocase. Nature 401, 485–489 (1999).
Brix, J., Rüdiger, S., Bukau, B., Schneider-Mergener, J. & Pfanner, N. Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J. Biol. Chem. 274, 16522–16530 (1999).
Abe, Y. et al. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell 100, 551–560 (2000).
Hill, K. et al. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395, 516–521 (1998).
Baker, K.P., Schaniel, A., Vestweber, D. & Schatz, G. A yeast mitochondrial outer membrane protein essential for protein import and cell viability. Nature 348, 605–609 (1990).
Dietmeier, K. et al. Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature 388, 195–200 (1997).
Model, K. et al. Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat. Struct. Biol. 8, 361–370 (2001).
Schneider, H. et al. Targeting of the master receptor MOM19 to mitochondria. Science 254, 1659–1662 (1991).
Rapaport, D. & Neupert, W. Biogenesis of Tom40, core component of the TOM complex of mitochondria. J. Cell Biol. 146, 321–331 (1999).
Taylor, R.D., McHale, B.J. & Nargang, F.E. Characterization of Neurospora crassa Tom40-deficient mutants and effect of specific mutations on Tom40 assembly. J. Biol. Chem. 278, 765–775 (2003).
Wiedemann, N. et al. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424, 565–571 (2003).
Gratzer, S. et al. Mas37p, a novel receptor subunit for protein import into mitochondria. J. Cell Biol. 129, 25–34 (1995).
Ryan, M.T., Müller, H. & Pfanner, N. Functional staging of ADP/ATP carrier trans-location across the outer mitochondrial membrane. J. Biol. Chem. 274, 20619–20627 (1999).
Kozjak, V. et al. An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J. Biol. Chem. 278, 48520–48523 (2003).
Paschen, S.A. et al. Evolutionary conservation of biogenesis of β-barrel membrane proteins. Nature 426, 862–866 (2003).
Gentle, I., Gabriel, K., Beech, P., Waller, R. & Lithgow, T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164, 19–24 (2004).
Milenkovic, D. et al. Sam35 of the mitochondrial protein sorting and assembly machinery is a peripheral outer membrane protein essential for cell viability. J. Biol. Chem. 279, 22781–22785 (2004).
Waizenegger, T. et al. Tob38, a novel essential component in the biogenesis of β-barrel proteins of mitochondria. EMBO Rep. 5, 704–709 (2004).
Wiedemann, N et al. Biogenesis of the protein import channel Tom40 of the mitochondrial outer membrane: intermembrane space components are involved in an early stage of the assembly pathway. J. Biol. Chem. 279, 18188–18194 (2004).
Hoppins, S.C. & Nargang, F.E. The Tim8–Tim13 complex of Neurospora crassa functions in the assembly of proteins into both mitochondrial membranes. J. Biol. Chem. 279, 12396–12405 (2004).
Smith, M., Hicks, S., Baker, K. & McCauley, R. Rupture of the mitochondrial outer membrane impairs porin assembly. J. Biol. Chem. 269, 28460–28464 (1994).
Voulhoux, R., Bos, M.P., Geurtsen, J., Mols, M. & Tommassen, J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299, 262–265 (2003).
Genevrois, S., Steeghs, L., Roholl, P., Letesson, J.J. & van der Ley, P. The Omp85 protein of Neisseria meningitidis is required for lipid export to the outer membrane. EMBO J. 22, 1780–1789 (2003).
Voulhoux, R. & Tommassen, J. Omp85, an evolutionarily conserved bacterial protein involved in outer-membrane-protein assembly. Res. Microbiol. 155, 129–135 (2004).
Schäfer, U., Beck, K. & Müller, M. Skp, a molecular chaperone of Gram-negative bacteria, is required for the formation of soluble periplasmic intermediates of outer membrane proteins. J. Biol. Chem. 274, 24567–24574 (1999).
Harms, N. et al. The early interaction of the outer membrane protein PhoE with the periplasmic chaperone Skp occurs at the cytoplasmic membrane. J. Biol. Chem. 276, 18804–18811 (2001).
Soll, J. & Schleiff, E. Protein import into chloroplasts. Nat. Rev. Mol. Cell Biol. 5, 198–208 (2004).
Eckart, K. et al. A Toc75-like protein import channel is abundant in chloroplasts. EMBO Rep. 3, 557–562 (2002).
Meisinger, C. et al. The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev. Cell 7, 61–71 (2004).
Sogo, L.F. & Yaffe, M.P. Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J. Cell Biol. 126, 1361–1373 (1994).
Boldogh, I.R. et al. A protein complex containing Mdm10p, Mdm12p, and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery. Mol. Biol. Cell 14, 4618–4627 (2003).
Pfanner, N. et al. Role of ATP in mitochondrial protein import: conformational alteration of a precursor protein can substitute for ATP requirement. J. Biol. Chem. 263, 4049–4051 (1988).
Armstrong, L.C., Saenz, A.J. & Bornstein, P. Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J. Cell. Biochem. 74, 11–22 (1999).
Wang, X. et al. Metaxin is required for tumor necrosis factor-induced cell death. EMBO Rep. 2, 628–633 (2001).
Ryan, M.T. Chaperones: inserting β-barrels into membranes. Curr. Biol. 14, R207–R209 (2004).
Shaw, J.M. & Nunnari, J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 12, 178–184 (2002).
Dimmer, K.S. et al. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 847–853 (2002).
Acknowledgements
We are grateful to G. Schatz, F. Paltauf, S.D. Kohlwein, J. Soll, R.E. Jensen, K.Mihara, I. Gentle and M.T. Ryan for discussion and support in clarifying the nomenclature of the SAM subunits according to the rules of the Saccharomyces genome database (SGD). Work of the authors' laboratories was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 388, Gottfried Wilhelm Leibniz Programme, Max Planck Research Award, Alexander von Humboldt Foundation, Bundesministerium für Bildung und Forschung, the Fonds der Chemischen Industrie and the Australian Research Council.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pfanner, N., Wiedemann, N., Meisinger, C. et al. Assembling the mitochondrial outer membrane. Nat Struct Mol Biol 11, 1044–1048 (2004). https://doi.org/10.1038/nsmb852
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb852
This article is cited by
-
Pan-Cancer Analysis of the Prognostic and Immunological Role of TOMM40 to Identify Its Function in Breast Cancer
Biochemical Genetics (2024)
-
Transcriptional responses of Candida glabrata biofilm cells to fluconazole are modulated by the carbon source
npj Biofilms and Microbiomes (2020)
-
Atomic structure of human TOM core complex
Cell Discovery (2020)
-
Structural characterisation of a full-length mitochondrial outer membrane TOM40 preprotein translocase: implications for its interaction with presequence peptides
European Biophysics Journal (2019)
-
Characterization of the targeting signal in mitochondrial β-barrel proteins
Nature Communications (2016)