Abstract
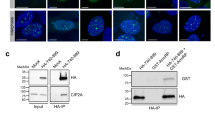
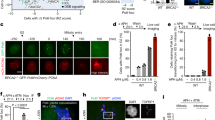
The tumor suppressor BRCA1 has an important function in the maintenance of genomic stability. Increasing evidence suggests that BRCA1 regulates cell cycle checkpoints and DNA repair after DNA damage. However, little is known about its normal function in the absence of DNA damage. Here we show that BRCA1 interacts and colocalizes with topoisomerase IIα in S phase cells. Similar to cells treated with the topoisomerase IIα inhibitor ICRF-193, BRCA1-deficient cells show lagging chromosomes, indicating a defect in DNA decatenation and chromosome segregation. More directly, BRCA1 deficiency results in defective DNA decatenation in vitro. Finally, topoisomerase IIα is ubiquitinated in a BRCA1-dependent manner, and topoisomerase IIα ubiquitination correlates with higher DNA decatenation activity. Together these results suggest an important role of BRCA1 in DNA decatenation and reveal a previously unknown function of BRCA1 in the maintenance of genomic stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108, 171–182 (2002).
Scully, R. & Livingston, D.M. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature 408, 429–432 (2000).
Kerr, P. & Ashworth, A. New complexities for BRCA1 and BRCA2. Curr. Biol. 11, R668–R676 (2001).
Brodie, S.G. & Deng, C.X. BRCA1-associated tumorigenesis: what have we learned from knockout mice? Trends Genet. 17, S18–S22 (2001).
Xu, X. et al. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat. Genet. 28, 266–271 (2001).
Xu, X. et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 22, 37–43 (1999).
Scully, R. et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 90, 425–435 (1997).
Scully, R. et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88, 265–275 (1997).
Agostinho, M. et al. Human topoisomerase IIalpha: targeting to subchromosomal sites of activity during interphase and mitosis. Mol. Biol. Cell 15, 2388–2400 (2004).
Niimi, A., Suka, N., Harata, M., Kikuchi, A. & Mizuno, S. Co-localization of chicken DNA topoisomerase IIalpha, but not beta, with sites of DNA replication and possible involvement of a C-terminal region of alpha through its binding to PCNA. Chromosoma 110, 102–114 (2001).
Yu, X., Chini, C.C., He, M., Mer, G. & Chen, J. The BRCT domain is a phospho-protein binding domain. Science 302, 639–642 (2003).
Manke, I.A., Lowery, D.M., Nguyen, A. & Yaffe, M.B. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302, 636–639 (2003).
Wang, J.C. DNA topoisomerases. Annu. Rev. Biochem. 65, 635–692 (1996).
Ishida, R. et al. Inhibition of intracellular topoisomerase II by antitumor bis(2,6-dioxopiperazine) derivatives: mode of cell growth inhibition distinct from that of cleavable complex-forming type inhibitors. Cancer Res. 51, 4909–4916 (1991).
Clarke, D.J., Johnson, R.T. & Downes, C.S. Topoisomerase II inhibition prevents anaphase chromatid segregation in mammalian cells independently of the generation of DNA strand breaks. J. Cell Sci. 105, 563–569 (1993).
Yarden, R.I., Pardo-Reoyo, S., Sgagias, M., Cowan, K.H. & Brody, L.C. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat. Genet. 30, 285–289 (2002).
Lucas, I., Germe, T., Chevrier-Miller, M. & Hyrien, O. Topoisomerase II can unlink replicating DNA by precatenane removal. EMBO J. 20, 6509–6519 (2001).
Marini, J.C., Miller, K.G. & Englund, P.T. Decatenation of kinetoplast DNA by topoisomerases. J. Biol. Chem. 255, 4976–4979 (1980).
Poljak, L. & Kas, E. Resolving the role of topoisomerase II in chromatin structure and function. Trends Cell Biol. 5, 348–354 (1995).
Taagepera, S., Rao, P.N., Drake, F.H. & Gorbsky, G.J. DNA topoisomerase II alpha is the major chromosome protein recognized by the mitotic phosphoprotein antibody MPM-2. Proc. Natl Acad. Sci. USA 90, 8407–8411 (1993).
Baer, R. & Ludwig, T. The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr. Opin. Genet. Dev. 12, 86–91 (2002).
Starita, L.M. & Parvin, J.D. The multiple nuclear functions of BRCA1: transcription, ubiquitination and DNA repair. Curr. Opin. Cell Biol. 15, 345–350 (2003).
Salmena, L., Lam, V., McPherson, J.P. & Goldenberg, G.J. Role of proteasomal degradation in the cell cycle-dependent regulation of DNA topoisomerase IIalpha expression. Biochem. Pharmacol. 61, 795–802 (2001).
Bachant, J., Alcasabas, A., Blat, Y., Kleckner, N. & Elledge, S.J. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9, 1169–1182 (2002).
Azuma, Y., Arnaoutov, A. & Dasso, M. SUMO-2/3 regulates topoisomerase II in mitosis. J. Cell Biol. 163, 477–487 (2003).
Meyer, K.N. et al. Cell cycle-coupled relocation of types I and II topoisomerases and modulation of catalytic enzyme activities. J. Cell Biol. 136, 775–788 (1997).
Wang, R.H., Yu, H. & Deng, C.X. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc. Natl Acad. Sci. USA 101, 17108–17113 (2004).
Gimenez-Abian, J.F. et al. Premitotic chromosome individualization in mammalian cells depends on topoisomerase II activity. Chromosoma 109, 235–244 (2000).
Deming, P.B. et al. The human decatenation checkpoint. Proc. Natl Acad. Sci. USA 98, 12044–12049 (2001).
Scully, R. et al. Location of BRCA1 in human breast and ovarian cancer cells. Science 272, 123–126 (1996).
Chen, J.J., Silver, D., Cantor, S., Livingston, D.M. & Scully, R. BRCA1, BRCA2, and Rad51 operate in a common DNA damage response pathway. Cancer Res. 59, 1752s–1756s (1999).
Rappold, I., Iwabuchi, K., Date, T. & Chen, J. Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J. Cell Biol. 153, 613–620 (2001).
Lou, Z., Minter-Dykhouse, K., Wu, X. & Chen, J. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 421, 957–961 (2003).
Lou, Z., Chini, C.C., Minter-Dykhouse, K. & Chen, J. Mediator of DNA damage checkpoint protein 1 regulates BRCA1 localization and phosphorylation in DNA damage checkpoint control. J. Biol. Chem. 278, 13599–13602 (2003).
Acknowledgements
We thank R. Baer (Columbia University) for assisting us with the ubiquitination assay and J. Wood for providing suggestions and proofreading of this manuscript. This work is supported by grants from US National Institutes of Health (NIH RO1 CA89239 and CA92312 to J.C.). J.C. is a recipient of a Department of Defense (DOD) breast cancer career development award (DAMD17-02-1-0472). Z.L. is a recipient of a DOD breast cancer fellowship award (DAMD17-03-1-0610).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lou, Z., Minter-Dykhouse, K. & Chen, J. BRCA1 participates in DNA decatenation. Nat Struct Mol Biol 12, 589–593 (2005). https://doi.org/10.1038/nsmb953
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb953
This article is cited by
-
DNA damage repair system in C57BL/6 J mice is evolutionarily stable
BMC Genomics (2021)
-
Dysregulated G2 phase checkpoint recovery pathway reduces DNA repair efficiency and increases chromosomal instability in a wide range of tumours
Oncogenesis (2021)
-
Targeting nucleolin for better survival in diffuse large B-cell lymphoma
Leukemia (2018)
-
Roles of eukaryotic topoisomerases in transcription, replication and genomic stability
Nature Reviews Molecular Cell Biology (2016)
-
RNF168 and USP10 regulate topoisomerase IIα function via opposing effects on its ubiquitylation
Nature Communications (2016)