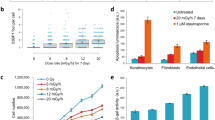
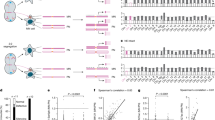
Abstract
Characterization of the direct effects of DNA-damaging agents shows how DNA lesions lead to specific mutations. Yet, serum from Hiroshima survivors, Chernobyl liquidators and radiotherapy patients can induce a clastogenic effect on naive cells, showing indirect induction of genomic instability that persists years after exposure. Such indirect effects are not restricted to ionizing radiation, as chemical genotoxins also induce heritable and transmissible genomic instability phenotypes. Although such indirect induction of genomic instability is well described, the underlying mechanism has remained enigmatic. Here, we show that mouse embryonic stem cells exposed to γ-radiation bear the effects of the insult for weeks. Specifically, conditioned media from the progeny of exposed cells can induce DNA damage and homologous recombination in naive cells. Notably, cells exposed to conditioned media also elicit a genome-destabilizing effect on their neighbouring cells, thus demonstrating transmission of genomic instability. Moreover, we show that the underlying basis for the memory of an insult is completely dependent on two of the major DNA cytosine methyltransferases, Dnmt1 and Dnmt3a. Targeted disruption of these genes in exposed cells completely eliminates transmission of genomic instability. Furthermore, transient inactivation of Dnmt1, using a tet-suppressible allele, clears the memory of the insult, thus protecting neighbouring cells from indirect induction of genomic instability. We have thus demonstrated that a single exposure can lead to long-term, genome-destabilizing effects that spread from cell to cell, and we provide a specific molecular mechanism for these persistent bystander effects. Collectively, our results impact the current understanding of risks from toxin exposures and suggest modes of intervention for suppressing genomic instability in people exposed to carcinogenic genotoxins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Azzam E, De Toledo SM, Spitz DR, Little JB . (2002). Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res 62: 5436–5442.
Bender MA, Gooch PC . (1962). Persistent chromosome aberrations in irradiated human subjects. Radiat Res 16: 44–53.
Borowczyk E, Mohan KN, D'Aiuto L, Cirio MC, Chaillet JR . (2009). Identification of a region of the DNMT1 methyltransferase that regulates the maintenance of genomic imprints. Proc Natl Acad Sci USA 106: 20806–20811.
Burr KL, Robinson JI, Rastogi S, Boylan MT, Coates PJ, Lorimore SA et al. (2010). Radiation-induced delayed bystander-type effects mediated by hemopoietic cells. Radiat Res 173: 760–768.
Chang WP, Little JB . (1992). Delayed reproductive death as a dominant phenotype in cell clones surviving X-irradiation. Carcinogenesis 13: 923–928.
Chen L . (1991). Direct identification of the active-site nucleophile in a DNA (cytosine-5)-methyltransferase. Biochemistry 30: 11018–11025.
Chen RZ, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R . (1998). DNA hypomethylation leads to elevated mutation rates. Nature 395: 89–93.
Dickey JS, Baird BJ, Redon CE, Sokolov MV, Sedelnikova OA, Bonner WM . (2009). Intercellular communication of cellular stress monitored by gamma-H2AX induction. Carcinogenesis 30: 1686–1695.
Dolinoy D, Weidman JR, Waterland RA, Jirtle RL . (2006). Maternal genistein alters coat color and protects avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect 114: 567–572.
Engelward BP, Dreslin A, Christensen J, Huszar D, Kurahara C, Samson L . (1996). Repair-deficient 3-methyladenine DNA glycosylase homozygous mutant mouse cells have increased sensitivity to alkylation-induced chromosome damage and cell killing. EMBO J 15: 945–952.
Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD et al. (2010). Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci 13: 423–430.
Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T . (2006). DNA Repair and Mutagenesis, 2nd edn. ASM Press: Washington, DC.
Goldberg AD, Allis CD, Bernstein E . (2007). Epigenetics: A landscape takes shape. Cell 128: 635–638.
Goldberg Z, Lehnert BE . (2002). Radiation-induced effects in unirradiated cells: a review and implications in cancer. Int J Oncol 21: 337–349.
Hermann A, Gowher H, Jeltsch A . (2004). Biochemistry and biology of the mammalian DNA methyltransferases. Cell Mol Life Sci 61: 2571–2587.
Hoeijmakers HJ . (2009). DNA damage aging and cancer. N Engl J Med 361: 1475–1485.
Huang L, Kim PM, Nickoloff JA, Morgan WF . (2007). Targeted and nontargeted effects of low-dose ionizing radiation on delayed genomic instability in human cells. Cancer Res 67: 1099–1104.
Huo L, Nagasawa H, Little JB . (2001). HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations. Radiat Res 156: 521–525.
Kadhim M, Macdonald DT, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG . (1992). Transmission of chromosomal instability after plutonium α-particle irradiation. Nature 355: 738–740.
Kim M, Trinh BN, Long TI, Oghamian S, Laird PW . (2004). Dnmt1 deficiency leads to enhanced microsatellite instability in mouse embryonic stem cells. Nucleic Acids Res 32: 5742–5749.
Koturbash I . (2006). Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene 25: 4267–4275.
Kovalchuk O, Baulch JE . (2008). Epigenetic changes and nontargeted radiation effects—is there a link? Environ Mol Mutagen 49: 16–25.
Lei H, Oh SP, Okano M, Juttermann R, Goss KA, Jaenisch R et al. (1996). De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development 122: 3195–3205.
Lewis DA, Mayhugh BM, Qin Y, trott K, Mendonca MS . (2001). Production of delayed death and neoplastic transformation in CGL1 cells by radiation-induced bystander effects. Radiat Res 156: 251–258.
Li E . (1992). Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69: 915–926.
Limoli CL, Day JP, Ward JF, Morgan WF . (1998). Induction of chromosome aberrations and delayed genomic instability by photochemical processes. Photochem Photobiol 67: 233–238.
Little J . (2003). Genomic instability and bystander effects: a historical perspective. Oncogene 22: 6978–6987.
Little JB, Gorgojo L, Vetrovs H . (1990). Delayed appearance of lethal and specific gene mutations in irradiated mammalian cells. IntJ Radiat Oncol Biol Phys 19: 1425–1429.
Lorimore SA, Coates PJ, Wright EG . (2003). Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene 22: 7058–7069.
Lorimore SA, McIlrath JM, Coates PJ, Wright EG . (2005). Chromosomal instability in unirradiated hemopoietic cells resulting from a delayed in vivo bystander effect of gamma radiation. Cancer Res 65: 5668–5673.
Maltseva DV, Gromova ES . (2009). Interaction of murine Dnmt3a with DNA containing O6-methylguanine. Biochemistry 75: 173–181.
Maxwell CA, Fleisch MC, Costes SV, Erickson AC, Boissiere A, Gupta R et al. (2008). Targeted and nontargeted effects of ionizing radiation that impact genomic instability. Cancer Res 68: 8304–8311.
Morgan WF . (2003). Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res 159: 581–596.
Morgan WF, Sowa MB . (2005). Effects of ionizing radiation in nonirradiated cells. Proc Natl Acad Sci USA 102: 14127–14128.
Mortusewicz O, Schermelleh L, Walter J, Cardoso MC, Leonhardt H . (2005). Recruitment of DNA methyltransferase I to DNA repair sites. Proc Natl Acad Sci USA 102: 8905–8909.
Mothersill C, Crean M, Lyons M, McSweeney J, Mooney R, O'Reilly J et al. (1998). Expression of delayed toxicity and lethal mutations in the progeny of human cells surviving exposure to radiation and other environmental mutagens. Int J Radiat Biol 74: 673–680.
Mothersill C, O'Malley KO, Seymour CB . (2002). Characterisation of a bystander effect induced in human tissue explant cultures by low LET radiation. Radiat Prot Dosimetry 99: 163–167.
Mothersill C, Seymour C . (2001). Radiation-induced bystander effects: past history and future directions. Radiat Res 155: 759–767.
Mothersill C, Seymour C . (2005). Radiation-induced bystander effects: are they good, bad or both? Med Confl Surviv 21: 101–110.
Mothersill C, Seymour C . (2006). Radiation-induced bystander effects: evidence for an adaptive response to low dose exposures? Dose Response 4: 283–290.
Mothersill C, Seymour CB . (2004). Radiation-induced bystander effects—implications for cancer. Nat Rev Cancer 4: 158–164.
Mothersill C, Smith RW, Agnihotri N, Seymour CB . (2007). Characterization of a radiation-induced stress response communicated in vivo between zebrafish. Environ Sci Technol 41: 3382–3387.
Nagar S, Smith LE, Morgan WF . (2003). Characterization of a novel epigenetic effect of ionizing radiation: the death-inducing effect. Cancer Res 63: 324–328.
Nagasawa H, Little JB . (1992). Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res 52: 6394–6396.
Okano M, Bell DW, Haber DA, Li E . (1999). DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99: 247–257.
Olive PL, Banath JP . (2006). The comet assay: a method to measure DNA damage in individual cells. Nat: Protoc 1: 23–29.
Pampfer S, Streffer C . (1989). Increased chromosome aberration levels in cells from mouse fetuses after zygote X-irradiation. Int J Radiat Biol 55: 85–92.
Pant G, Kamada N . (1977). Chromosome aberrations in normal leukocytes induced by the plasma of exposed individuals. Hiroshima J Med Sci 26: 149–154.
Rodier F, Coppe J-P, Patil CK, Hoeijmakers WAM, Munoz DP, Raza SR et al. (2009). Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 1272.
Rugo R . (2005). A single acute exposure to a chemotherapeutic agent induces hyper-recombination in distantly descendant cells and in their neighbors. Oncogene 24: 5016–5025.
Seymour CB, Mothersill C . (2004). Radiation-induced bystander effects—implications for cancer. Nat Rev Cancer 4: 158–164.
Stein R . (1982). Clonal inheritance of the pattern of DNA methylation in mouse cells. Proc Natl Acad Sci USA 79: 61–65.
Tsumura A . (2006). Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a, Dnmt3b. Genes Cells 11: 805–814.
Watson GE, Lorimore SA, Macdonald DA, Wright EG . (2000). Chromosomal instability in unirradiated cells induced in vivo by a bystander effect of ionizing radiation. Cancer Res 60: 5608–5611.
Yang G, Mei T, Yuan H, Zhang W, Chen L, Xue J . (2008). Bystander/abscopal effects induced in intact Arabidopsis seeds by low-energy heavy-ion radiation. Radiat Res 170: 372–380.
Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK . (2000). Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci USA 97: 2099–2104.
Acknowledgements
We thank M Okano and E Li for MTase-deficient ES cells. This work was supported primarily by NIH Grant RO1-CA83876-8, NIH/NIAID CMCR U19 AI068021, with partial support from the Department of Energy DE-FG02-05ER64053. We thank the NIEHS Center for Environmental Health Sciences (P30-ES002109), the Cancer Research Center Flow Cytometry Facility and Debby Pheasant for technical support. We thank Benjamin Greenberger, Paavni Komanduri and Werner Olipitz for their contributions.
Author contributions: RER designed and performed all experiments with support from TY; KNM and JRC engineered the dox controllable Dnmt1 system and performed western analysis; BPE prepared the paper with support from JTM; BPE and JSG oversaw the study design. All authors discussed the results and commented on the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rugo, R., Mutamba, J., Mohan, K. et al. Methyltransferases mediate cell memory of a genotoxic insult. Oncogene 30, 751–756 (2011). https://doi.org/10.1038/onc.2010.480
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.480
Keywords
This article is cited by
-
Differential molecular response in mice and human thymocytes exposed to a combined-dose radiation regime
Scientific Reports (2022)
-
Epigenetics in radiation-induced fibrosis
Oncogene (2015)
-
Intraclonal recovery of ‘slow clones’—a manifestation of genomic instability: Are mitochondria the key to an explanation?
Radiation and Environmental Biophysics (2014)