Abstract
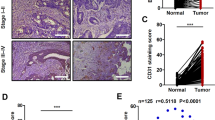
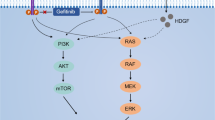
Experimental and clinical studies positively correlate expression of vascular endothelial growth factor (VEGF)-C in cancer cells with accelerated tumor progression and/or unfavorable clinical outcome. However, many aspects of tumor-promoting activity of VEGF-C and consequences of its downregulation for tumor progression remain poorly understood. To clarify these points, we created a set of VEGF receptor 3-positive lung carcinoma A549 and colon carcinoma HCT116 cell sublines with stable repression of VEGF-C synthesis. Analysis of the behavior of these cells revealed multiple effects of VEGF-C downregulation, which, in addition to deceleration of cell proliferation and invasion in vitro and inhibition of lymphangiogenesis in tumor and surrounding tissues observed earlier, included previously undescribed effects, in particular, partial restoration of epithelial phenotype, reduction in the percentage of tumor-initiating cells (cancer stem cells) in the cell population and inhibition of metastasis of orthotopic lung cancer xenografts to other lung lobes. These results are consistent with the idea of high potentiality of VEGF-C as a cancer drug target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y . (2003). Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer 97: 457–464.
Bahram F, Claesson-Welsh L . (2010). VEGF-mediated signal transduction in lymphatic endothelial cells. Pathophysiology 17: 253–261.
Chen Y, Jiang L, She F, Tang N, Wang X, Li X et al. (2010). Vascular endohelial growth factor-C promotes the growth and invasion of gallbladder cancer via an autocrine mechanism. Mol Cell Biochem 345: 77–89.
Gu Y, Qi X, Guo S . (2008). Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: a retrospective study of 61 cases. Clin Exp Metastasis 25: 717–725.
Guarino M, Rubino B, Ballabio G . (2007). The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39: 305–318.
Gupta PB, Chaffer CL, Weinberg RA . (2009). Cancer stem cells: mirage or reality? Nat Med 15: 1010–1012.
He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T et al. (2002). Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 94: 819–825.
Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B et al. (2006). Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 66: 8065–8075.
Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED et al. (2007). Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol 213: 374–383.
Jackson MW, Roberts JS, Heckford SE, Ricciardelli C, Stahl J, Choong C et al. (2002). A potential autocrine role for vascular endothelial growth factor in prostate cancer. Cancer Res 62: 854–859.
Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E et al. (1996). A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15: 290–298.
Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y et al. (1997). Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 16: 3898–3911.
Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K et al. (2001). A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA 98: 12677–12682.
Karpanen T, Heckman C, Keskitalo S, Jeltsch M, Ollila H, Alitalo K . (2006). Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J 20: 1462–1472.
Khromova NV, Kopnin PB, Stepanova EV, Agapova LS, Kopnin BP . (2009). p53 hot-spot mutants increase tumor vascularization via ROS-mediated activation of the HIF1/VEGF-A pathway. Cancer Lett 276: 143–151.
Kodama M, Kitadai Y, Tanaka M, Kuwai T, Tanaka S, Oue N et al. (2008). Vascular endothelial growth factor C stimulates progression of human gastric cancer via both autocrine and paracrine mechanisms. Clin Cancer Res 14: 7205–7214.
Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R et al. (2001). Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 20: 672–682.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715.
Miyazaki T, Okada N, Ishibashi K, Ogata K, Ohsawa T, Ishiguro T et al. (2008). Clinical significance of plasma level of vascular endothelial growth factor-C in patients with colorectal cancer. Jpn J Clin Oncol 38: 839–843.
Orlandini M, Spreafico A, Bardelli M, Rocchigiani M, Salameh A, Nucciotti S et al. (2006). Vascular endothelial growth factor-D activates VEGFR-3 expressed in osteoblasts inducing their differentiation. J Biol Chem 281: 17961–17967.
Polyak K, Weinberg RA . (2009). Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9: 265–273.
Shibuya M, Claesson-Welsh L . (2006). Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 312: 549–560.
Singh A, Settleman J . (2010). EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29: 4741–4751.
Skobe M, Brown LF, Tognazzi K, Ganju RK, Dezube BJ, Alitalo K et al. (1999). Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J Invest Dermatol 113: 1047–1053.
Strizzi L, Catalano A, Vianale G, Orecchia S, Casalini A, Tassi G et al. (2001). Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol 193: 468–475.
Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY et al. (2006). The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell 9: 209–223.
Su JL, Chen PS, Chien MH, Chen PB, Chen YH, Lai CC et al. (2008). Further evidence for expression and function of the VEGF-C/VEGFR-3 axis in cancer cells. Cancer Cell 13: 557–560.
Tammela T, Alitalo K . (2010). Lymphangiogenesis: molecular mechanisms and future promise. Cell 140: 460–476.
Thiery JP, Acloque H, Huang RY, Nieto MA . (2009). Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890.
Timoshenko AV, Rastogi S, Lala PK . (2007). Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br J Cancer 97: 1090–1098.
Wang X, Fu X, Hoffman RM . (1992). A patient-like metastasizing model of human lung adenocarcinoma constructed via thoracotomy in nude mice. Anticancer Res 12: 1399–1401.
Wu C, Alman BA . (2008). Side population cells in human cancers. Cancer Lett 268: 1–9.
Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I et al. (2010). Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol 188: 115–130.
Yamashita T, Uramoto H, Onitsuka T, Ono K, Baba T, So T et al. (2010). Association between lymphangiogenesis-/micrometastasis- and adhesion-related molecules in resected stage I NSCLC. Lung Cancer 70: 320–328.
Yamaura T, Murakami K, Doki Y, Sugiyama S, Misaki T, Yamada Y et al. (2000). Solitary lung tumors and their spontaneous metastasis in athymic nude mice orthotopically implanted with human non-small cell lung cancer. Neoplasia 2: 315–324.
Zeisberg M, Neilson EG . (2009). Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119: 1429–1437.
Acknowledgements
The work was supported by grants from Russian Fund for Basic Research (BK, # 08-04-00252), PROTEK-CRC Program for Basic Research in Oncology and Russian Federal Program ‘Scientific and pedagogical personnel of innovative Russia’ (BK, # 02.740.11.0085).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Khromova, N., Kopnin, P., Rybko, V. et al. Downregulation of VEGF-C expression in lung and colon cancer cells decelerates tumor growth and inhibits metastasis via multiple mechanisms. Oncogene 31, 1389–1397 (2012). https://doi.org/10.1038/onc.2011.330
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.330
Keywords
This article is cited by
-
A zebrafish HCT116 xenograft model to predict anandamide outcomes on colorectal cancer
Cell Death & Disease (2022)
-
RETRACTED ARTICLE: Lymphatic metastasis-related TBL1XR1 enhances stemness and metastasis in gastric cancer stem-like cells by activating ERK1/2-SOX2 signaling
Oncogene (2021)
-
High expression of PTPRM predicts poor prognosis and promotes tumor growth and lymph node metastasis in cervical cancer
Cell Death & Disease (2020)
-
An orally administered DNA vaccine targeting vascular endothelial growth factor receptor-3 inhibits lung carcinoma growth
Tumor Biology (2016)
-
In vivo and in vitro effect of hepatocarcinoma lymph node metastasis by upregulation of Annexin A7 and relevant mechanisms
Tumor Biology (2016)