Abstract
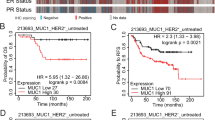
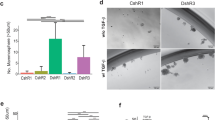
Human epidermal growth factor receptor 2 (HER2)/Neu is overexpressed in 20–30% of breast cancers and associated with aggressive phenotypes and poor prognosis. For deciphering the role of HER2/Neu in breast cancer, mouse mammary tumor virus (MMTV)-Her2/neu transgenic mice that develop mammary tumors resembling human HER2-subtype breast cancer have been established. Several recent studies have revealed that HER2/Neu is overexpressed in and regulates self renewal of breast tumor-initiating cells (TICs). However, in the MMTV-Her2/neu transgenic mouse model, the identity of TICs remains elusive, despite previous studies showing supportive evidence for existence of TICs in Her2/neu-induced mammary tumors. Through systematic screening and characterization, we identified that surface markers CD49f, CD61 and ESA were aberrantly overexpressed in Her2-overexpressing mammary tumor cells. Analysis of these markers and CD24 detected anomalous expansion of the luminal progenitor population in preneoplastic mammary glands of Her2/neu transgenic mice, indicating that aberrant luminal progenitors originated in Her2-induced mammary tumors. The combined markers, CD49f and CD61, further delineated the CD49fhighCD61high-sorted fraction as a TIC-enriched population, which displayed increased tumorsphere formation ability, enhanced tumorigenicity both in vitro and in vivo and drug resistance to pacitaxel and doxorubicin. Moreover, the TIC-enriched population manifested increased transforming growth factor-β (TGFβ) signaling and exhibited gene expression signatures of stemness, TGFβ signaling and epithelial-to-mesenchymal transition. Our findings that self-renewal and clonogenicity of TICs were suppressed by pharmacologically inhibiting the TGFβ signaling further indicate that the TGFβ pathway is vital for maintenance of the TIC population. Finally, we showed that the integrin-β3 (CD61) signaling pathway was required for sustaining active TGFβ signaling and self-renewal of TICs. We for the first time developed a technique to highly enrich TICs from mammary tumors of Her2/neu transgenic mice, unraveled their properties and identified the cooperative integrin-β3–TGFβ signaling axis as a potential therapeutic target for HER2-induced TICs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC et al. (2007). Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9: 201–209.
Biswas S, Criswell TL, Wang SE, Arteaga CL . (2006). Inhibition of transforming growth factor-beta signaling in human cancer: targeting a tumor suppressor network as a therapeutic strategy. Clin Cancer Res 12: 4142–4146.
Chen H, Pimienta G, Gu Y, Sun X, Hu J, Kim MS et al. (2010). Proteomic characterization of Her2/neu-overexpressing breast cancer cells. Proteomics 10: 3800–3810.
Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B et al. (2009). The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 138: 1083–1095.
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL et al. (2006). Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 66: 9339–9344.
Desgrosellier JS, Cheresh DA . (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10: 9–22.
Galliher AJ, Schiemann WP . (2006). Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res 8: R42.
Galliher AJ, Schiemann WP . (2007). Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res 67: 3752–3758.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1: 555–567.
Gu Y, Fu J, Lo P, Wang S, Wang Q, Chen H . (2011). The effect of B27 supplement on promoting in vitro propagation of Her2/neu-transformed mammary tumorspheres. J Biotech Res 3: 7–18.
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ . (1992). Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 89: 10578–10582.
Hanker L, Karn T, Ruckhaeberle E, Gaetje R, Solbach C, Schmidt M et al. (2010). Clinical relevance of the putative stem cell marker p63 in breast cancer. Breast Cancer Res Treat 122: 765–775.
Hynes NE, Jaggi R, Kozma SC, Ball R, Muellener D, Wetherall NT et al. (1985). New acceptor cell for transfected genomic DNA: oncogene transfer into a mouse mammary epithelial cell line. Mol Cell Biol 5: 268–272.
Ishikawa T, Nakagawa H . (2009). Human ABC transporter ABCG2 in cancer chemotherapy and pharmacogenomics. J Exp Ther Oncol 8: 5–24.
James D, Levine AJ, Besser D, Hemmati-Brivanlou A . (2005). TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132: 1273–1282.
Kalluri R, Weinberg RA . (2009). The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428.
Korkaya H, Paulson A, Iovino F, Wicha MS . (2008). HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 27: 6120–6130.
Kveiborg M, Flyvbjerg A, Eriksen EF, Kassem M . (2001). Transforming growth factor-beta1 stimulates the production of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in human bone marrow stromal osteoblast progenitors. J Endocrinol 169: 549–561.
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF et al. (2008). Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100: 672–679.
Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH et al. (2009). Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15: 907–913.
Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F et al. (2010). Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res 12: R21.
Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E . (2007). Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res 67: 8671–8681.
Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW et al. (2006). Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66: 6063–6071.
Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P et al. (2009). Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res 15: 2010–2021.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715.
Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N et al. (2007). Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA 104: 10069–10074.
Margadant C, Sonnenberg A . (2010). Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 11: 97–105.
Park JI, Lee MG, Cho K, Park BJ, Chae KS, Byun DS et al. (2003). Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene 22: 4314–4332.
Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM et al. (2004). alpha(v)beta(3) Integrin interacts with the transforming growth factor beta (TGFbeta) type II receptor to potentiate the proliferative effects of TGFbeta1 in living human lung fibroblasts. J Biol Chem 279: 37726–37733.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML et al. (2006). Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88.
Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J et al. (2007). Molecular definition of breast tumor heterogeneity. Cancer Cell 11: 259–273.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D et al. (2006). Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997.
Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE . (2008). The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res 68: 7711–7717.
Visvader JE . (2011). Cells of origin in cancer. Nature 469: 314–322.
Welm B, Behbod F, Goodell MA, Rosen JM . (2003). Isolation and characterization of functional mammary gland stem cells. Cell Prolif 36(Suppl 1): 17–32.
Wipff PJ, Rifkin DB, Meister JJ, Hinz B . (2007). Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323.
Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L . (2008). Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res 10: R10.
Yang C, Patel K, Harding P, Sorokin A, Glass II WF . (2007). Regulation of TGF-beta1/MAPK-mediated PAI-1 gene expression by the actin cytoskeleton in human mesangial cells. Exp Cell Res 313: 1240–1250.
Acknowledgements
We thank Dr Marai M Pena, Celestia Davis, Sara Johnson and Andrea Daamen for experimental assistance. This work was supported by the Elsa U Pardee Cancer Foundation grant (B94AFFAA), the American Cancer Society Research Award (RSG-10-067-01-TBE) to HC and NIH grant (3P20RR017698-08) to HC and QW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lo, PK., Kanojia, D., Liu, X. et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin–TGFβ signaling. Oncogene 31, 2614–2626 (2012). https://doi.org/10.1038/onc.2011.439
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.439
Keywords
This article is cited by
-
Rank ectopic expression in the presence of Neu and PyMT oncogenes alters mammary epithelial cell populations and their tumorigenic potential
Journal of Mammary Gland Biology and Neoplasia (2023)
-
KRT13 promotes stemness and drives metastasis in breast cancer through a plakoglobin/c-Myc signaling pathway
Breast Cancer Research (2022)
-
Integrins regulate stemness in solid tumor: an emerging therapeutic target
Journal of Hematology & Oncology (2021)
-
Use of constitutive and inducible oncogene-containing iPSCs as surrogates for transgenic mice to study breast oncogenesis
Stem Cell Research & Therapy (2021)
-
Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance
Oncogene (2021)