Abstract
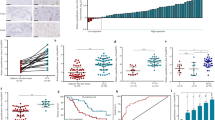
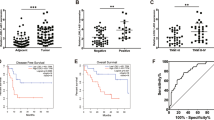
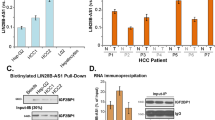
We have recently identified nc886 (pre-miR-886 or vtRNA2-1) as a novel type of non-coding RNA that inhibits activation of protein kinase R (PKR). PKR’s pro-apoptotic role through eukaryotic initiation factor 2 α (eIF2α) phosphorylation is well established in the host defense against viral infection. Paradoxically, some cancer patients have elevated PKR activity; however, its cause and consequence are not understood. Initially, we evaluated the expression of nc886, PKR and eIF2α in non-malignant cholangiocyte and cholangiocarcinoma (CCA) cells. nc886 is repressed in CCA cells and this repression is the cause of PKR’s activation therein. nc886 alone is necessary and sufficient for suppression of PKR via direct physical interaction. Consistently, artificial suppression of nc886 in cholangiocyte cells activates the canonical PKR/eIF2α cell death pathway, suggesting a potential significance of the nc886 suppression and the consequent PKR activation in eliminating pre-malignant cells during tumorigenesis. In comparison, active PKR in CCA cells does not induce phospho-eIF2α nor apoptosis, but promotes the pro-survival nuclear factor-κB pathway. Thus, PKR has a dual life or death role during tumorigenesis. Similarly to the CCA cell lines, nc886 tends to be decreased but PKR tends to be activated in our clinical samples from CCA patients. Collectively from our data, we propose a tumor surveillance model for nc886’s role in the PKR pathway during tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lee K, Kunkeaw N, Jeon SH, Lee I, Johnson BH, Kang GY et al. Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 2011; 17: 1076–1089.
Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007; 129: 1401–1414.
Nandy C, Mrazek J, Stoiber H, Grasser FA, Huttenhofer A, Polacek N . Epstein-barr virus-induced expression of a novel human vault RNA. J Mol Biol 2009; 388: 776–784.
Stadler PF, Chen JJ, Hackermuller J, Hoffmann S, Horn F, Khaitovich P et al. Evolution of vault RNAs. Mol Biol Evol 2009; 26: 1975–1991.
Alisi A, Ghidinelli M, Zerbini A, Missale G, Balsano C . Hepatitis C virus and alcohol: same mitotic targets but different signaling pathways. J Hepatol 2011; 54: 956–963.
Treppendahl MB, Qiu X, Sogaard A, Yang X, Nandrup-Bus C, Hother C et al. Allelic methylation levels of the non-coding VTRNA2-1 located on chromosome 5q31.1 predict outcome in AML. Blood 2012; 119: 206–216.
Garcia MA, Meurs EF, Esteban M . The dsRNA protein kinase PKR: virus and cell control. Biochimie 2007; 89: 799–811.
Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H et al. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR). Cancer Res 2002; 62: 2239–2243.
Scheuner D, Patel R, Wang F, Lee K, Kumar K, Wu J et al. Double-stranded RNA-dependent protein kinase phosphorylation of the alpha-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J Biol Chem 2006; 281: 21458–21468.
Li YJ, Zeng JM, Huang SF, Wang XZ, Zhao SQ, Bai WJ et al. Selective leukemia cell death by activation of the double-stranded RNA-dependent protein kinase PKR. Int J Mol Med 2011; 28: 215–222.
Prasad S, Ravindran J, Aggarwal BB . NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem 2010; 336: 25–37.
Mounir Z, Koromilas AE . Uncovering the PKR pathway's potential for treatment of tumors. Future Oncol 2010; 6: 643–645.
Gil J, Esteban M . Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 2000; 5: 107–114.
Pataer A, Raso MG, Correa AM, Behrens C, Tsuta K, Solis L et al. Prognostic significance of RNA-dependent protein kinase on non-small cell lung cancer patients. Clin Cancer Res 2010; 16: 5522–5528.
Haines GK, Panos RJ, Bak PM, Brown T, Zielinski M, Leyland J et al. Interferon-responsive protein kinase (p68) and proliferating cell nuclear antigen are inversely distributed in head and neck squamous cell carcinoma. Tumour Biol 1998; 19: 52–59.
Beretta L, Gabbay M, Berger R, Hanash SM, Sonenberg N . Expression of the protein kinase PKR in modulated by IRF-1 and is reduced in 5q-associated leukemias. Oncogene 1996; 12: 1593–1596.
Hii SI, Hardy L, Crough T, Payne EJ, Grimmett K, Gill D et al. Loss of PKR activity in chronic lymphocytic leukemia. Int J Cancer 2004; 109: 329–335.
Murad JM, Tone LG, de Souza LR, De Lucca FL . A point mutation in the RNA-binding domain I results in decrease of PKR activation in acute lymphoblastic leukemia. Blood Cells Mol Dis 2005; 34: 1–5.
Savinova O, Joshi B, Jagus R . Abnormal levels and minimal activity of the dsRNA-activated protein kinase, PKR, in breast carcinoma cells. Int J Biochem Cell Biol 1999; 31: 175–189.
Kim SH, Forman AP, Mathews MB, Gunnery S . Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene 2000; 19: 3086–3094.
Terada T, Maeta H, Endo K, Ohta T . Protein expression of double-stranded RNA-activated protein kinase in thyroid carcinomas: correlations with histologic types, pathologic parameters, and Ki-67 labeling. Hum Pathol 2000; 31: 817–821.
Kim SH, Gunnery S, Choe JK, Mathews MB . Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene 2002; 21: 8741–8748.
Haines GK, Cajulis R, Hayden R, Duda R, Talamonti M, Radosevich JA . Expression of the double-stranded RNA-dependent protein kinase (p68) in human breast tissues. Tumour Biol 1996; 17: 5–12.
Hiasa Y, Kamegaya Y, Nuriya H, Onji M, Kohara M, Schmidt EV et al. Protein kinase R is increased and is functional in hepatitis C virus-related hepatocellular carcinoma. Am J Gastroenterol 2003; 98: 2528–2534.
Blalock WL, Grimaldi C, Fala F, Follo M, Horn S, Basecke J et al. PKR activity is required for acute leukemic cell maintenance and growth: a role for PKR-mediated phosphatase activity to regulate GSK-3 phosphorylation. J Cell Physiol 2009; 221: 232–241.
Basu S, Panayiotidis P, Hart SM, He LZ, Man A, Hoffbrand AV et al. Role of double-stranded RNA-activated protein kinase in human hematological malignancies. Cancer Res 1997; 57: 943–947.
Rosenwald IB . Upregulated expression of the genes encoding translation initiation factors eIF-4E and eIF-2alpha in transformed cells. Cancer Lett 1996; 102: 113–123.
Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM . Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein beta isoform and up-regulation of the eukaryotic translation initiation factor 2alpha are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res 1996; 56: 4382–4386.
Rosenwald IB, Rhoads DB, Callanan LD, Isselbacher KJ, Schmidt EV . Increased expression of eukaryotic translation initiation factors eIF-4E and eIF-2 alpha in response to growth induction by c-myc. Proc Natl Acad Sci USA 1993; 90: 6175–6178.
Rosenwald IB, Hutzler MJ, Wang S, Savas L, Fraire AE . Expression of eukaryotic translation initiation factors 4E and 2alpha is increased frequently in bronchioloalveolar but not in squamous cell carcinomas of the lung. Cancer 2001; 92: 2164–2171.
Lobo MV, Martin ME, Perez MI, Alonso FJ, Redondo C, Alvarez MI et al. Levels, phosphorylation status and cellular localization of translational factor eIF2 in gastrointestinal carcinomas. Histochem J 2000; 32: 139–150.
Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T et al. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation 2004; 77: 446–451.
Tepsiri N, Chaturat L, Sripa B, Namwat W, Wongkham S, Bhudhisawasdi V et al. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J Gastroenterol 2005; 11: 2748–2753.
Yonglitthipagon P, Pairojkul C, Chamgramol Y, Mulvenna J, Sripa B . Up-regulation of annexin A2 in cholangiocarcinoma caused by Opisthorchis viverrini and its implication as a prognostic marker. Int J Parasitol 2010; 40: 1203–1212.
Lee TG, Tang N, Thompson S, Miller J, Katze MG . The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol Cell Biol 1994; 14: 2331–2342.
Onori P, DeMorrow S, Gaudio E, Franchitto A, Mancinelli R, Venter J et al. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int J Cancer 2009; 125: 565–576.
Datta B . MAPs and POEP of the roads from prokaryotic to eukaryotic kingdoms. Biochimie 2000; 82: 95–107.
Rowlands AG, Panniers R, Henshaw EC . The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J Biol Chem 1988; 263: 5526–5533.
Reichel PA, Merrick WC, Siekierka J, Mathews MB . Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature 1985; 313: 196–200.
Pavitt GD, Ramaiah KV, Kimball SR, Hinnebusch AG . eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev 1998; 12: 514–526.
Balachandran S, Barber GN . Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 2004; 5: 51–65.
Fabian JR, Kimball SR, Jefferson LS . Reconstitution and purification of eukaryotic initiation factor 2B (eIF2B) expressed in Sf21 insect cells. Protein Expr Purif 1998; 13: 16–22.
Jeon SH, Lee K, Lee KS, Kunkeaw N, Johnson BH, Holthauzen LMF et al. Characterization of the direct physical interaction of nc886, a cellular non-coding RNA, and PKR. FEBS Letters 2012 (in press, http://dx.doi.org/10.1016/j.febslet.2012.07.076).
Wek RC, Jiang HY, Anthony TG . Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 2006; 34 (Part 1): 7–11.
Novoa I, Zeng H, Harding HP, Ron D . Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 2001; 153: 1011–1022.
Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol 2003; 163: 767–775.
Ikegami T, Narayanan K, Won S, Kamitani W, Peters CJ, Makino S . Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog 2009; 5: e1000287.
Acknowledgements
We thank Dr Sopit Wongkham, Dr Kanlayanee Sawanyawisuth and Ms Sirinapa Sribenja (Khon Kaen University) for CCA cells and helpful discussion; Dr Inhan Lee (miRcore, MI) for helpful advice; Research Histology Core Laboratory Facility, University of Texas, MD Anderson Cancer Center (funded by the National Cancer Institute Grant # CA16672) for tissue preparation and IHC. This work was supported by a Research Scholar Grant, RSG-12-187-01—RMC from the American Cancer Society to YSL, by start-up funding from the Sealy Center for Cancer Biology at the University of Texas Medical Branch to YSL, by the Royal Golden Jubilee (RGJ) scholarship (PHD/0105/2550) of Thailand Research Fund (TRF) to NK and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0022022) to SHJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kunkeaw, N., Jeon, S., Lee, K. et al. Cell death/proliferation roles for nc886, a non-coding RNA, in the protein kinase R pathway in cholangiocarcinoma. Oncogene 32, 3722–3731 (2013). https://doi.org/10.1038/onc.2012.382
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.382
Keywords
This article is cited by
-
Vault RNAs: hidden gems in RNA and protein regulation
Cellular and Molecular Life Sciences (2021)
-
Nc886 is epigenetically repressed in prostate cancer and acts as a tumor suppressor through the inhibition of cell growth
BMC Cancer (2018)
-
nc886 is induced by TGF-β and suppresses the microRNA pathway in ovarian cancer
Nature Communications (2018)
-
Heritable DNA methylation marks associated with susceptibility to breast cancer
Nature Communications (2018)
-
Translational regulator eIF2α in tumor
Tumor Biology (2014)