Abstract
Study design:
Measurement of sympathetic effector organ responses to selective activation of muscle and skin nociceptors below lesion in spinal cord-injured (SCI) subjects.
Objectives:
To test whether selective noxious stimulation below lesion causes exaggerated sympathetic responses in human SCI.
Setting:
Prince of Wales Medical Research Institute, Australia.
Methods:
Twelve subjects (C5-T10, ASIA A-C), none of whom had sensation below the lesion, were included in the study. Selective stimulation of muscle or cutaneous nociceptors was produced by bolus injection of hypertonic (5%) saline into the tibialis anterior muscle or overlying skin and compared with non-noxious electrical stimulation of the abdominal wall. Cutaneous vasoconstrictor (photoelectric plethysmography) and sudomotor (skin conductance) responses, in addition to respiration, heart rate and continuous arterial pressure were monitored.
Results:
Electrical stimulation of the abdominal wall caused a significant increase in arterial pressure (31.8±6.1%). Conversely, intramuscular or subcutaneous injection of hypertonic saline caused no significant changes in blood pressure (−3.0±2.4%; −1.4±3.4%) heart rate, skin blood flow or sweat release.
Conclusions:
While hypertonic saline injected into muscle or skin induces strong pain, cutaneous vasoconstriction and sweat release in able-bodied subjects, we saw no evidence of exaggerated sympathoexcitation when these same noxious stimuli were delivered below lesion in subjects with SCI. This suggests that certain types of somatic noxious input may not trigger autonomic dysreflexia, and questions the concept that any painful stimuli originating below lesion can reliably trigger dysreflexia.
Similar content being viewed by others
Introduction
Autonomic dysreflexia (AD) is a medical emergency, clinically characterised by a massive increase in blood pressure. It is believed that the increase in blood pressure is initiated and maintained by a sympathetically mediated vasoconstriction in muscle, skin and splanchnic vascular beds.1 The increase in blood pressure can have dangerous consequences, including stroke and death. AD is triggered by stimuli originating below the level of lesion. Despite the existence of evidence linking non-noxious stimuli2 with AD, current dogma states that noxious stimuli are the primary (and sole) initiators of AD. Since visceral organ distension and other known triggers of AD may be associated with a subjective ‘noxious’ or unpleasant quality, it may be easy to attribute the episode of AD to a presumed noxious input rather than to other types of afferent input. The primary aim of this study was to test the hypothesis that selective activation of nociceptors in muscle and skin below lesion evokes a pronounced vasoconstriction, and hence, increases in blood pressure in subjects with spinal cord injury (SCI). Selective activation of muscle or cutaneous nociceptors was achieved by intramuscular or subcutaneous injections of hypertonic saline, which in able-bodied individuals causes a dull, diffuse ache or localized burning pain.3
Materials and methods
Subjects
Twelve subjects with SCI were investigated (Table 1), the majority having sustained injuries above T6. However, as AD has been reported to the level of T10,4 one subject (with a T10 lesion) was included in the sample. With the exception of two subjects (T6—(33 years post-injury) and T10), all subjects had experienced AD in the past relating to blocked indwelling and suprapubic urinary catheters. Patients with unstable blood pressure at rest, recent history of AD (<3 months) antihypertensive medication, any sensation in their legs, ongoing infection or other comorbidities were excluded. All participating subjects gave written (or oral and witnessed) informed consent, and the study received ethical approval from the Human Research Ethics Committee of The University of New South Wales. (Table 1)
Procedures
In all experiments, subjects rested with their eyes closed, in a semi-reclined position at a comfortable ambient temperature. Blood pressure was continuously monitored using noninvasive radial artery tonometry (CBM-7000, Colin Corp., Japan) and heart rate by standard Ag–AgCl ECG chest electrodes and recorded on a computer-based data acquisition system (PowerLab 16SP, ADInstruments, Bella Vista, Australia). Sweat release was assessed from changes in electrical conductance of the glabrous skin on the first and third fingers and toes (GSR amplifier, ADInstruments). Changes in pulsatile skin blood volume were monitored by infrared photo-plethysmography probes that are attached to the pads of the second finger and toe (IR Plethysmograph, ADInstruments). Respiration was recorded with a strain gauge transducer, attached to a strap around the chest (Pneumotrace, UFI, Morro Bay, CA, USA). Chart v5.5 (ADInstruments) was used for data acquisition and analysis.
Electrical stimulation
Following a resting baseline of approximately 10 min, standardized electrical stimulation was applied below lesion (abdominal wall) at unexpected times, the aim being to investigate the ability of the isolated spinal cord to generate reflex increases in blood pressure in response to stimulation of cutaneous (myelinated) afferents.5 Cutaneous electrical stimulation (3–10 mA, 0.2–1.0-ms pulses at 20 Hz, 1 s trains) was delivered by 1-cm Ag–AgCl surface electrodes applied bilaterally (4 cm apart) to the abdominal wall, midway between the umbilicus and pubis. Stimuli were delivered from a software-controlled optically isolated source (Stimulus Isolator, PowerLab, ADInstruments). Stimulus intensity was increased until clear blood pressure responses were observed or up to a maximum current of 10 mA (1 ms) in 10 subjects.
Nociceptive stimuli
After recording a resting baseline period of approximately 10 min, a 23G cannula was inserted ∼1 cm into the belly of the tibialis anterior muscle; another was inserted subdermally into the overlying skin ∼5 mm away. Intramuscular (0.5 ml) or subcutaneous (0.2 ml) bolus injections of hypertonic (5%) saline (hNaCl), which reaches peak pain around 1.5 min and lasts around 5–10 min in able-bodied subjects,3 were made at unexpected times and in quasirandom order.
Analysis
Instantaneous heart rate was calculated from the echocardio graph. Skin blood flow from the toe ipsilateral to injections was high-pass filtered (0.5 Hz) and pulse amplitude (peak-trough) calculated. Mean values for systolic blood pressure, heart rate and skin blood flow were calculated from 15-s sample blocks. Effector organ responses were normalized to allow comparisons between individuals. A 5-min period was analysed, comprising a 1-min baseline prior to the injection and a 4-min period following the injection. The greatest peak deviation (+/−) in blood pressure from baseline (%) was defined as the peak blood pressure response.
Statistics
Paired t-tests were used to compare peak systolic blood pressure responses between electrical stimulation, muscle and skin injections, and baseline values. The Kruskal–Wallis test was used to determine whether any changes in systolic blood pressure, heart rate and skin blood flow changes postinjections were significant compared with baseline. A probability level of less than 5% (one-tailed) was regarded as significant.
Results
Blood pressure
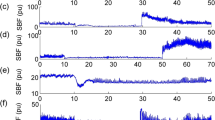
There were no significant increases in blood pressure following intramuscular or subdermal injections of hNaCl compared with baseline (Figure 1). Increases in peak systolic blood pressure following electrical stimulation (mean 31.8±s.e. 6.1%) were significantly elevated when compared with baseline (P=<0.001). Muscle (−3.0±2.4%) and skin injections (−1.4±3.4%) (Figure 2) were not significantly elevated when compared with baseline. As indicated in Figure 3, there were no significant trends between peak blood pressure responses and time after injury for electrical stimulation, intramuscular or subdermal injections (R2=0.046; R2=0.003; R2=0.003) correlates (−0.21; 0.06; −0.06), or between peak blood pressure responses and ASIA score or level of injury.
This figure shows the response of systolic blood pressure (%) to intramuscular (top) and subdermal (bottom) injections into the tibialis anterior muscle and overlying skin (n=12; n=11). Intramuscular and subdermal injections of hypertonic saline, which cause strong pain in able-bodied subjects, did not cause any statistically significant increases in systolic blood pressure.
This figure shows the peak systolic blood pressure responses (%) to cutaneous electrical stimulation (myelinated afferents) versus muscle and skin (nociceptive afferents). Error bars show 95% confidence interval of mean values. There was a significant increase in blood pressure following electrical stimulation when compared with baseline. Muscle and skin injection values were not significantly different from baseline.
This figure shows the peak response of systolic blood pressure (%) to abdominal electrical stimulation, intramuscular and subdermal injections into the tibialis anterior muscle and overlying skin (n=12; n=11). There appeared to be a poor relationship between peak blood pressure responses and time after injury for the different stimuli.
Heart rate
There were no statistically significant changes in heart rate following muscle or skin injections (3.7% decrease; 1.8% decrease; median values measured 90 s after intramuscular and subdermal injections) (Figure 4).
Skin blood flow
There were no statistically significant changes in skin blood flow (9.4% increase; 3.7% decrease; median values measured 90 s after intramuscular and subdermal injections) (Figure 5). In addition, there were negligible changes in skin potential (sweat release).
Discussion
Triggers for AD may come from both noxious and non-noxious sources.1 However, a common definition found in the literature for the cause of AD resembles the following: ‘…any stimulus that might cause pain in a person without spinal cord injury is capable of triggering AD.’.6, 7 Our study has demonstrated that in a population of spinal cord-injured subjects who are capable of becoming dysreflexic, stimuli that cause strong pain in able-bodied subjects did not trigger autonomic dysreflexia. The use of such a definition, which excludes non-noxious trigger factors, may prove to be problematic in a clinical environment. For example, attributing an episode of AD to an assumed noxious stimulus (for example, ingrown toenails) may allow insidious trigger, such as heterotrophic ossification to go untreated, especially if the dysreflexic response is mild.
The concept of a noxious input as being a reliable initiator of AD may not hold true. For example, while pressure ulcers may be quite painful in intact individuals,8 and while there are reported cases of AD being associated with pressure sores,9 clinical experience does not support the concept that most spinal patients who have pressure ulcers consistently and continually suffer from constant AD; in other words, the presence of an assumed continuous nociceptive stimulus does not necessarily translate into a continual hypertensive state.
Anecdotal evidence from the authors' clinical experience has suggested that even known nociceptive stimuli may not be reliable triggers of AD. For example, a patient with a known clinical history of AD relating to blocked indwelling urinary catheters (T6 ASIA A) experienced a spiral fracture of his right femur after a fall. Despite his predisposition to AD, regular observations (including blood pressure) were stable following the fall and he did not experience any symptoms of AD following the fracture. This contrasts with another documented case study where a similar stimulus was implicated in an episode of AD.10 This suggests that while noxious inputs may be capable of triggering AD under certain conditions, it may not be a reliable trigger for all individuals.
Indeed, non-noxious stimuli have been implicated in triggering AD. For example, vibration of the penis is not noxious, yet the use of vibroejaculation for sperm retrieval in individuals with SCI has been documented to trigger AD.11 Moreover, light stroking of the skin is capable of inducing AD in spinalized rats, as is colonic distension below levels that evoke signs of pain in intact rats.2 Visceral organ distension has been associated with AD, for example bladder tapping/pushing and blocked urinary catheters, leading to distension of the bladder.12, 13 Similarly, AD has been experimentally associated with colonic distension in animals, anorectal procedures and rectal stimulation associated with lower bowel evacuation in humans.2, 14, 15 The use of topical anaesthetic cream was unable to prevent AD during functional electrical stimulation and it was concluded that ‘…mechanisms other than skin nociception are operative in functional electrical stimulation-induced AD.16
In the spinal cord-injured population, it has been proposed that an increase in blood pressure of at least 20% with a combined visualized vasoconstriction is necessary to suggest an episode of AD.1 As hNaCl-induced pain rapidly builds and peaks in able-bodied subjects, particularly in light of the case study of dysreflexia evoked by intramuscular injection of antibiotics,6 one would expect that sympathetic responses should be seen within the first few minutes following injections. We did not see an exaggerated sympathetic response in spinal cord-injured subjects during this time frame. The implication of these findings is that pure nociceptive input from lower limb skin and skeletal muscle may not reliably trigger autonomic dysreflexia.
It is generally accepted that a dysreflexic episode will last as long as the afferent stimulus is present.13 The blood pressure and effector organ responses following injections of hNaCl seen in this study were transient, inconsistent and not reflective of the duration and intensity of nociception seen in able-bodied subjects. The largest increase in blood pressure (16%) following subdermal injection of hNaCl was shown by a T6 (ASIA A) subject (Figure 3), yet this outlying response only lasted a short time.
Since increases in blood pressure were seen following electrical stimulation and not following hNaCl injections, this study suggests that the dysreflexic response may be related to stimulation of non-nociceptive afferents. For example, while painful mechanical stimuli can trigger AD,2 it may be that this is caused by coactivation of larger diameter mechano-sensitive afferents rather than nociceptive afferents. Again, since hNaCl specifically activates group III and IV afferents,17 perhaps the modest decreases in skin blood flow following intramuscular injections may be related to excitation of large diameter myelinated fibres during the bolus injection phase, rather than nociceptive.
It has been suggested that the location of trigger stimuli and time after SCI may also be critical factors for an episode of AD to occur. Animal studies have found that noxious and innocuous stimulation of somatic segments projecting to caudal lumbar segments had the potential to decrease ongoing renal sympathetic nerve activity in acutely injured rats; however, this relationship appeared to diminish as the spinal injury progressed from acute to chronic.18 However, no such relationship was evident in our study with chronic SCI subjects (>15 years post injury); some showing increases in blood pressure and some showing decreases in blood pressure in response to skin and muscle nociception (Figure 3). Again, while all subjects showed increases in blood pressure following electrical stimulation of the abdominal wall, we found no significant relationship between peak blood pressure responses and time after injury. However, since the study by Krassioukov et al.18 used a different stimulus (mechanical pinching), a comparison between our trend findings and the previous study may not be applicable.
The use of a definition similar to: ‘…any stimulus that might cause pain in a person without SCI is capable of triggering AD’ does not adequately address the diverse range of stimuli (including non-noxious stimuli) that can cause AD. We suggest that a more useful and accurate definition of AD trigger stimuli should reflect the following: …the AD reaction has the potential to be ‘provoked by peripheral afferent (noxious and non-noxious) stimulation below the lesion level reaching the isolated spinal cord.’1
Limitations
The nociceptive stimuli used in our study were confined to skeletal muscle and skin over a limb below lesion. However, since hNaCl causes pain in able-bodied subjects irrespective of where it is injected, while possible, it is unlikely that hNaCl injections delivered at different dermatomal levels would produce differing effects.18
Conclusions
Selective nociceptive stimulation of skin and muscle overlying tibialis anterior in spinal cord-injured subjects did not cause an exaggerated sympathetic response indicative of autonomic dysreflexia, despite these same stimuli causing strong sensations of pain in able-bodied subjects. This study suggests that not all noxious stimuli may be reliable triggers of AD. To assume that any stimulus that might cause pain in a person without SCI is capable of triggering AD, may allow one to overlook underlying non-noxious trigger factors. When deducing potential trigger stimuli for autonomic dysreflexia, we reaffirm the evidence in the literature and strongly encourage investigators to also consider non-noxious stimuli as potential causes.
References
Karlsson AK . Autonomic dysreflexia. Spinal Cord 1999; 37: 383–391.
Marsh DR, Weaver LC . Autonomic dysreflexia, induced by noxious or innocuous stimulation, does not depend on changes in dorsal horn substance p. J Neurotrauma 2004; 21: 817–828.
Henderson LA, Bandler R, Gandevia SC, Macefield VG . Distinct forebrain activity patterns during deep versus superficial pain. Pain 2006; 120: 286–296.
Franklin DJ . Back to the basics. N Engl J Med 1999; 341: 2099–2100.
Brown R, Engel S, Wallin BG, Elam M, Macefield V . Assessing the integrity of sympathetic pathways in spinal cord injury. Auton Neurosci 2007; 22: 22.
Blackmer J . Rehabilitation medicine: 1. Autonomic dysreflexia.[see comment]. CMAJ 2003; 169: 931–935.
Selcuk B, Inanir M, Kurtaran A, Sulubulut N, Akyuz M . Autonomic Dysreflexia After Intramuscular Injection in Traumatic Tetraplegia. Am J Phys Med Rehabil 2004; 83: 61–64.
Roth RS, Lowery JC, Hamill JB . Assessing persistent pain and its relation to affective distress, depressive symptoms, and pain catastrophizing in patients with chronic wounds: a pilot study. Am J Phys Med Rehabil 2004; 83: 827–834.
Teasell RW, Arnold JM, Krassioukov A, Delaney GA . Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000; 81: 506–516.
Beard J, Wade W, Barber D . Sacral insufficiency stress fracture as etiology of positional autonomic dysreflexia: case report. Paraplegia 1996; 34: 173–175.
Elliott SL . Problems of sexual function after spinal cord injury. Prog Brain Res 2006; 152: 387–399.
Fagius J, Karhuvaara S . Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension 1989; 14: 511–517.
Karlsson A-K . Autonomic dysfunction in spinal cord injury: clinical presentation of symptoms and signs. Prog Brain Res 2006; 152: 1–8.
Kirshblum S, House J, O′Connor K . Silent autonomic dysreflexia during a routine Bowel program in persons with traumatic spinal cord injury: a preliminary study. Arch Phys Med Rehabil 2002; 83: 1774–1776.
Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA . Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res 2006; 152: 245–263.
Matthews JM, Wheeler GD, Burnham RS, Malone LA, Steadward RD . The effects of surface anaesthesia on the autonomic dysreflexia response during functional electrical stimulation. Spinal Cord 1997; 35: 647–651.
Capra NF, Ro JY . Human and animal experimental models of acute and chronic muscle pain: intramuscular algesic injection. Pain 2004; 110: 3–7.
Krassioukov AV, Johns DG, Schramm LP . Sensitivity of sympathetically correlated spinal interneurons, renal sympathetic nerve activity, and arterial pressure to somatic and visceral stimuli after chronic spinal injury. J Neurotrauma 2002; 19: 1521–1529.
Acknowledgements
We appreciate the assistance of Danielle Burton for recruiting the subjects who participated in this study and the advice of Dr Gunnar Wasner (Christian-Albrechts University of Kiel, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burton, A., Brown, R. & Macefield, V. Selective activation of muscle and skin nociceptors does not trigger exaggerated sympathetic responses in spinal-injured subjects. Spinal Cord 46, 660–665 (2008). https://doi.org/10.1038/sc.2008.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.33
Keywords
This article is cited by
-
Electrode position markedly affects knee torque in tetanic, stimulated contractions
European Journal of Applied Physiology (2016)
-
Consistent interindividual increases or decreases in muscle sympathetic nerve activity during experimental muscle pain
Experimental Brain Research (2014)
-
Biphasic effects of tonic stimulation of muscle nociceptors on skin sympathetic nerve activity in human subjects
Experimental Brain Research (2012)
-
Pressor response to passive walking-like exercise in spinal cord-injured humans
Clinical Autonomic Research (2009)
-
Input–output relationships of a somatosympathetic reflex in human spinal injury
Clinical Autonomic Research (2009)