Abstract
Study design:
Measurement of haemodynamic responses and cutaneous blood flow during an inspiratory-capacity apnoea following spinal cord injury (SCI).
Objective:
To assess the capacity of the sympathetic nervous system to respond to a cardiovascular challenge following SCI.
Setting:
Prince of Wales Medical Research Institute, Australia.
Subjects:
Thirteen spinal cord injured subjects with injuries ranging from C5-T8 and eight able-bodied control subjects.
Methods:
Continuous blood pressure, an electrocardiogram, respiration and cutaneous blood flow were recorded during a static maximum inspiratory breath-hold for 40 s.
Results:
On average, systolic blood pressure decreased 26% from baseline in the spinal group during the breath-hold and remained below baseline throughout the entire apnoeic period. Heart rate in this group had an initial decrease from baseline but quickly increased throughout the breath-hold, being 17% above baseline in the recovery period. Systolic pressure in the control group decreased 12% from baseline at the beginning of the breath-hold but quickly stabilized for the remainder of the apnoea, with heart rate initially decreasing 22% and remaining below baseline throughout the breath-hold.
Conclusion:
A maximal inspiratory breath-hold, which is known to cause a sustained increase in muscle sympathetic nerve activity, is a simple test to perform in supine spinal cord-injured subjects, and provides information on the capacity of muscle and splanchnic vasoconstrictor activity to increase blood pressure in SCI. A sustained decrease in blood pressure, coupled with an increase in heart rate, infers interruption of sympathetic vasoconstrictor pathways to muscle and splanchnic vascular beds.
Similar content being viewed by others
Introduction
A major consequence of spinal cord injury (SCI) is loss of sympathetic control of blood pressure (BP) through interruption of descending vasomotor tracts. While reflexly evoked increases in sympathetic vasoconstrictor activity can lead to dangerous elevations of BP, a phenomenon known as autonomic dysreflexia,1, 2, 3, 4 the absence of resting vasoconstrictor drive leads to a low resting BP and the inability to compensate for postural challenges—orthostatic hypotension.5
Orthostatic hypotension that affects individuals with SCI due to interruption of sympathetic vasoconstrictor outflow from supraspinal control is exhibited by a sudden decrease in BP associated with tachycardia upon postural changes.3 Sympathetic nervous system dysfunction,4, 6 altered baroreflex function7 and cardiovascular deconditioning8 are some of the factors thought to be responsible for orthostatic hypotension following SCI. In able-bodied individuals, the same postural change causes an increase in BP with virtually no change in heart rate (HR).9
We know that in the able-bodied during an inspiratory-capacity apnoea with the glottis closed and the inspiratory muscles relaxed, there is a reflex increase in muscle sympathetic nerve activity (MSNA) that is sustained for the period of the apnoea.10 Cutaneous sympathetic nerve activity increases transiently at the onset and offset of the inflation, but not throughout the entire apnoea. There is also a fall in systolic and diastolic pressures during the first half of the manoeuvre, with a return to baseline values in the second half of the manoeuvre.11 It is believed that the increase in MSNA is due to unloading of the low pressure baroreceptors.
This extends from our recent work in which we used indirect measures of cutaneous sympathetic nerve activity (cutaneous vasoconstriction and sweat release) to assess the integrity of sympathetic pathways through and below a spinal lesion. We looked at reflex increases in sympathetic nerve activity (SNA) and found skin blood flow to be a robust measurement of cutaneous sympathetic activation. Continuing on from this, we are now looking at an indirect measure of MSNA (and splanchnic) by using continuous BP and HR recordings to assess the capacity of the sympathetic nervous system to respond to cardiovascular challenges following an SCI.
Methods
Experiments were performed on 13 male SCI subjects; 6 paraplegic subjects (T3–T8; ASIA A–C) and 7 quadriplegic subjects (C5–C8; ASIA A–C) and in 8 healthy able-bodied controls. All subjects gave written (or oral and witnessed) informed consent, and the study received ethical approval from the Human Research Ethics Committees of The University of New South Wales and Prince of Wales Hospital.
Procedure
Subjects were studied resting in a semi-reclined position, with BP monitored non-invasively using radial artery tonometry (CBM-7000, Colin Corp., Japan) and an electrocardiogram (ECG) via standard Ag-AgCl ECG chest leads. Changes in skin blood volume, reflecting changes in skin blood flow, were recorded via infrared pulse plethysmograph probes. These were placed on the pad of a finger only for the able-bodied individuals, and on a finger and toe for the spinal-injured subjects (infrared plethysmograph, ADInstruments, Castle Hill, New South Wales, Australia). Respiratory movements were recorded by a strain gauge transducer attached to a strap around the chest (Pneumotrace, Morro Bay, CA, USA).
After recording a 10 min resting period a sustained inflation of the lungs was brought about by a maximal voluntary inhalation (to inspiratory-capacity) and maintained for at least 30 s by closure of the glottis. This was repeated a number of times in most subjects after an adequate rest period.
Analysis
Analysis of all data was performed by computer, which for each subject found the R waves of the ECG and computed systolic and diastolic pressures and calculated mean pressure and HR. Changes in skin blood flow were measured from the pulse amplitude taken from the raw plethysmography trace. The raw plethysmography trace was digitally filtered and an amplitude channel derived from this.
For a total of 50 R-waves systolic, diastolic and mean pressures, HR and skin blood flow were measured, with an additional 12 R-waves being measured during recovery. Averages were also calculated over a 10 s baseline prior to the apnoea, and over 10 s periods before and after the end of the manoeuvre, and the peak response measured as the lowest point of the BP and HR response. The paired t-test was used to show statistical significance between the able-bodied and spinal groups. A probability level of less than 5% (one tailed) was regarded as significant.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research, and all experiments were conducted with the understanding and consent of each subject.
Results
Experimental records from an able-bodied subject are shown in Figure 1. BP showed a precipitous fall at the beginning of the static lung inflation but rapidly recovered, remaining close to baseline for the remainder of the apnoea. HR increased during the inflation phase but quickly decreased and remained below baseline for the entire manoeuvre. Cutaneous blood flow in the hand decreased in the initial stage of the apnoea, increased after ∼10 s and then showed a secondary fall after ∼20 s. Records from a subject with SCI are shown in Figure 2. This subject sustained a C5 lesion 4 1/2 months previously and could still perform the maximum inspiratory apnoea and generate a large fall in intrathoracic pressure—as evidenced by the decrease in diastolic and systolic pressures. Unlike the able-bodied subject, the fall in BP in this subject not only remained well below baseline for the entire apnoea, but also took considerable time to recover after the resumption of breathing. Like the control subject, HR in this subject increased during the inflation phase and decreased at the onset of the static phase. However, it then showed a progressive increase throughout the apnoea. Perfusion in both the hand and foot decreased quickly and remained below baseline for the entire breath-hold; recovery of cutaneous flow was rapid upon resumption of breathing.
Blood pressure (radial arterial tonometry), heart rate and skin blood flow (infrared (IR) plethysmography, finger) responses to an inspiratory-capacity apnoea in an able-bodied subject. The black bar represents the period of the manoeuvre. Note the initial brief fall in blood pressure coincides with the initial fall in skin blood flow.
Blood pressure (BP), heart rate and skin blood flow (recorded from a finger and toe) responses to an inspiratory-capacity apnoea in a C5 ASIA A spinal patient. The black bar represents the period of the manoeuvre. Note the sustained fall in BP and skin blood flow and the progressive increase in heart rate.
Average changes in systolic, diastolic and mean pressures, as well as HR, for both control and SCI groups are shown in Table 1. Note that the resting systolic and diastolic pressures were lower in subjects with SCI. The average duration of the inspiratory-capacity apnoea was 37±4 s for the able-bodied and 38±4 s for the SCI groups.
Blood pressure and heart rate responses in able-bodied and spinal groups
In the able-bodied group, BP rapidly fell during the apnoea, reaching its nadir within 10 s. On average, the maximal falls in systolic and diastolic pressure were 16±6 mm Hg (12%) and 14±4 mm Hg (19%), respectively. In the SCI group, systolic pressure fell by 31±8 mm Hg (26%) and diastolic by 17±6 mm Hg (26%). The peak fall in mean BP was significantly greater in the SCI group than in the able-bodied group (P=0.01; paired t-test); the same was true for the mean BP during the static phase (10 s prior to the end of the manoeuvre; P=0.002). Importantly, systolic and diastolic pressures remained low for the duration of the apnoea in the SCI group, although in four subjects—C5 ASIA C, T5 ASIA A, T5 ASIA B, T8 ASIA C—BP did recover towards the end of the manoeuvre (presumably due to their increase in HR). Conversely, diastolic pressure in the able-bodied group recovered during the apnoea, showing some overshoot in the static phase of the manoeuvre; systolic pressure was slightly lower than baseline, resulting in a lower pulse pressure during the manoeuvre.
Heart rate showed the greatest difference in the two groups. In the control group, HR not only dropped by 16±2 b.p.m. (22%) at its lowest point, it was still well below baseline by the end of the apnoea. In the SCI group, HR also fell (10±5 b.p.m., 13%) at the beginning of the apnoea, but in 9 of the 13 subjects there was a rapid reversal after 10 s that led to HR showing a linear increase during the remainder of the apnoea, with the other 4 subjects having an HR increase closer to the end of the manoeuvre; during the recovery period, HR remained elevated, reaching a maximum of 13±5 b.p.m. (17%) above baseline.
Cutaneous blood flow in able-bodied and spinal groups
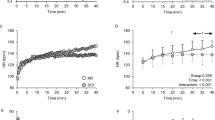
For the able-bodied group, skin blood flow in the hand decreased from baseline by almost half at the beginning of the apnoea, returning to baseline levels within 15 s (Figure 3). Towards the end of the apnoea, there was a further decrease in flow, which returned to control levels within the recovery period. However, in the SCI group perfusion in both the hand and the foot not only fell rapidly in the initial stage of the apnoea, but also remained low for the duration of the apnoea. Data from the foot are shown in Figure 3.
Average data from eight able-bodied subjects (filled circles) and 13 spinal cord-injured subjects (open circles) at rest, during an inspiratory-capacity apnoea and during recovery. The dashed lines represent the standard errors. The numbers on the abscissa refer to the number of R waves. The black bar represents the period of the manoeuvre. Skin blood flow was recorded from the finger of able-bodied subjects and data from the toe are included from the spinal cord-injured subjects.
Blood pressure and heart rate responses in paraplegics and quadriplegics
Average data from the SCI patients were further compared after dividing the group into those with cervical lesions (C5–C8, n=7) and those with thoracic lesions (T3–T8, n=6). As illustrated in Figures 4a and b, the fall in arterial pressure was more pronounced for the quadriplegic group, with the paraplegics showing some recovery during the manoeuvre. Resting HR was lower in the quadriplegics, but the initial increase during the inflation phase—and subsequent fall—was similar in the two groups. However, HR showed a greater increase during the static phase for the paraplegics.
Average data from six paraplegic (filled circles) and seven quadriplegic subjects (open circles) at rest, during an inspiratory-capacity apnoea and during recovery. The dashed lines represent the standard errors. The numbers on the abscissa refer to the number of R waves. The black bar represents the period of the manoeuvre. Skin blood flow was recorded from the fingers of both groups.
Cutaneous blood flow in paraplegics and quadriplegics
There were no differences in the two groups: cutaneous blood flow in the hand (Figure 4d) and foot (not shown) fell sharply at the beginning of the apnoea and remained at ∼50% of control for the duration of the manoeuvre.
Discussion
We have shown for the first time that SCI compromises the capacity of the cardiovascular system to respond to a maximal inspiratory breath-hold (inspiratory-capacity apnoea). Whereas in able-bodied subjects, the initial fall in BP at the onset of the manoeuvre was followed by a compensatory increase in diastolic pressure, both systolic and diastolic pressures remained low in the SCI group. Moreover, while the sustained hypotension led to a compensatory increase in HR in the SCI group—presumably due to a baroreflex-mediated withdrawal of cardiac vagal tone—there was no increase in the control subjects. This, coupled with the sustained fall in arterial pressure, provides differentiation in the response of able-bodied and SCI individuals to this manoeuvre and thereby presents as a possible additional test for assessing cardiovascular capacity in SCI.
Blood pressure and heart rate responses during inspiratory-capacity apnoea
We know that BP falls precipitously at the beginning of a maximal inspiratory breath-hold. Presumably, this is due to the reduction in stroke volume because the high intrathoracic pressure has reduced venous return to the heart.11 The reduction in HR may be due to reflex effects brought about by the fall in BP and changes of central venous pressure.10 As noted in the Introduction, it has been previously shown that—in able-bodied subjects—a maximal inspiratory breath-hold against a closed glottis (inspiratory muscles relaxed) causes a sustained increase in SNA to the muscle vascular bed.10, 11 This increase in MSNA—and its absence in SCI—explains the BP responses that were found in our study.
The BP responses we found in the spinal-injured group are what we would expect to find after SCI, given that in complete and incomplete lesions the descending sympathetic drive to the muscle vascular beds have often been interrupted.12 We know from microneurographic recordings in SCI that MSNA is absent at rest13 and is not evoked during an inspiratory-capacity apnoea (Macefield et al., unpublished observations). Due to this lack of sympathetic control—and the fact that the vagus nerve is still intact—the only means available to counteract the fall in arterial pressure during the manoeuvre is to increase HR. Indeed, HR was well above baseline levels during the static phase and continued to rise throughout the apnoea, presumably due to withdrawal of vagal tone to the heart. However, it would appear (Figures 3a–c) that this compensatory tachycardia is not sufficient to elevate BP to baseline levels.
Cutaneous blood flow during inspiratory-capacity apnoea
We know that skin sympathetic nerve activity (SSNA) does not show a sustained increase during the apnoea in able-bodied subjects11 and spinal subjects (Macefield et al., unpublished observations), but changes in skin blood flow during this manoeuvre had never been reported in the literature. We were surprised at the skin blood flow changes we observed in both the able-bodied and SCI groups: while a transient increase in SSNA would account for the initial fall in flow in the able-bodied subjects it could not account for the fall in the spinal subjects. Moreover, one would not expect skin blood flow to remain low given the lack of sustained cutaneous sympathetic nerve activity, yet blood flow remained low in the SCI group and showed a transient increase in the able-bodied group. We suggest that the apparent cutaneous vasoconstriction observed in the SCI group is no more than the expected behaviour of passive blood vessels exposed to a low arterial pressure and hence a low perfusion pressure. Conversely, for the able-bodied subjects, the increase in BP would cause an increase in perfusion pressure and hence an increase in skin blood flow. We are at a loss to explain why this increase reverted to a fall towards the end of the apnoea, other than to speculate that the secondary fall in HR may contribute to a reduction in perfusion (Figure 3d).
Limitations
One might expect that the production of a large inspiratory effort would be greatly compromised in the quadriplegics, given that their intercostal muscles are paralysed. Accordingly, the fall in intrathoracic pressure and, hence, the fall in BP generated during an inspiratory-capacity apnoea may reasonably be expected to be lower in the quadriplegics. Despite this, the hypotension was greater in these individuals, arguing against respiratory muscle weakness as being a limiting factor in their capacity to perform the manoeuvre. Apparently, the diaphragm alone is sufficient to generate an adequate response.
Conclusions
A maximal inspiratory breath-hold—which is known to cause a sustained increase in MSNA in able-bodied subjects—is a simple test to perform in supine spinal cord-injured subjects, and provides information on the capacity of muscle (and splanchnic) vasoconstrictor activity to increase BP. Using non-invasive monitoring of BP and HR, we are able to assess the capacity of the sympathetic nervous system to respond to a cardiovascular challenge: a sustained decrease in BP, coupled with an increase in HR, infers interruption of sympathetic vasoconstrictor pathways to muscle (and splanchnic) vascular beds. This may be a useful and simple assessment tool to use in the acute stage of SCI to determine the degree of hypotension an individual may suffer when moving into an upright position for the first time.
References
Silver JR . Early autonomic dysreflexia. Spinal Cord 2000; 38: 229–233.
Hamad GG, Merrell RC . Pheochromocytoma in quadriplegia. Curr Surg 2002; 59: 206–210.
Mathias CJ . Orthostatic hypotension and paroxysmal hypertension in humans with high spinal cord injury. Prog Brain Res 2006; 152: 231–243.
Mathias CJ, Frankel HL . Autonomic disturbances in spinal cord lesions. In: Mathias CJ, Bannister R (eds). Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. 4th edn. Oxford University Press: Oxford, 2002, pp 494–513.
Teasell RW, Arnold JM, Krassioukov A, Delaney GA . Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000; 81: 506–516.
Wallin BG, Stjernberg L . Sympathetic activity in man after spinal cord injury. Outflow to skin below the lesion. Brain 1984; 107 (Part 1): 183–192.
Wecht JM, De Meersman RE, Weir JP, Spungen AM, Bauman WA . Cardiac autonomic responses to progressive head-up tilt in individuals with paraplegia. Clin Auton Res 2004; 13: 433–438.
Vaziri ND . Nitric oxide in microgravity-induced orthostatic intolerance: relevance to spinal cord injury. J Spinal Cord Med 2003; 26: 5–11.
Claydon VE, Steeves JD, Krassioukov A . Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 2005; 44: 341–351.
Macefield VG, Wallin GB . Effects of static lung inflation on sympathetic activity in human muscle nerves at rest and during asphyxia. J Auton Nerv Syst 1995; 53: 148–156.
Macefield VG . Sustained activation of muscle sympathetic outflow during static lung inflation depends on a high intrathoracic pressure. J Auton Nerv Syst 1998; 68: 135–139.
Brown R, Engel S, Wallin GB, Elam M, Macefield V . Assessing the integrity of sympathetic pathways in spinal cord injury. Auton Neurosci Basic Clin 2007; 134: 61–68.
Stjernberg L, Blumberg H, Wallin BG . Sympathetic activity in man after spinal cord injury. Outflow to muscle below the lesion. Brain 1986; 109: 695–715.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, R., Macefield, V. Assessing the capacity of the sympathetic nervous system to respond to a cardiovascular challenge in human spinal cord injury. Spinal Cord 46, 666–672 (2008). https://doi.org/10.1038/sc.2008.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.35
Keywords
This article is cited by
-
Severity of autonomic dysfunction in patients with complete spinal cord injury
Clinical Autonomic Research (2012)
-
A systematic review of exercise as a therapeutic intervention to improve arterial function in persons living with spinal cord injury
Spinal Cord (2011)