Abstract
Originally identified as a B-cell marker, expression of the cell surface molecule CD24 has meanwhile been observed in a variety of human malignancies. It appears to function as a ligand of P-Selectin, an adhesion molecule that is present in activated platelets and endothelial cells. We aimed to determine the rate of CD24 expression in our nonsmall cell lung cancer (NSCLC) collection and to clarify its correlation with clinicopathological parameters including patients' survival. A total of 89 NSCLC were analysed immunohistochemically using a monoclonal CD24 antibody (clone 24C02) and a standard detection system (LSAB, DAKO) on NSCLC tissue microarrays (TMA). The staining was semiquantitatively scored (0, 1+, 2+, 3+) and grouped into high (2+, 3+)- and low (0, 1+)-level expression for statistical analysis. A high level of CD24 expression was observed in 45% of the cases, preferentially adenocarcinomas. Patients whose tumours had a high CD24 expression showed a significantly shorter median survival time of 23 months vs 38 months (P=0.033, log-rank test). Similarly tumour, grading, nodal status and clinical stage were significant prognostic markers in univariate survival analysis. Importantly, in the Cox regression-based multivariate analysis, CD24 expression (P=0.025) together with tumour stage (P=0.006) and grade (P=0.011) proved to be independent prognostic parameters. We hypothesise that the decreased survival of NSCLC patients with strongly CD24-positive tumours is related to an enhanced propensity of haematogenous metastasis formation, which might be P-Selectin mediated.
Similar content being viewed by others
Main
Lung cancer is a major cause of death from neoplastic malignancy in the Western world. In the USA alone, 169 400 new cases are expected for the year 2002 with 154 900 expected deaths from this disease (Jemal et al, 2002). In the last 20 years, the mortality rate could be lowered only by 6% because of improved therapies. So far, the most promising approach appears to be primary and secondary prevention (Henschke et al, 1999).
The molecular biology of nonsmall cell lung cancer (NSCLC), the largest lung cancer subgroup, is under intense international investigation in order to characterise new molecular marker genes which might be helpful in diagnosis or therapy. A gene that recently has gained new interest is CD24. It is a small heavily glycosylated mucin-like glycosyl-phosphatidylinositol(GPI)-linked cell surface protein (Pirruccello and Le Bien, 1986; Fischer et al, 1990; Akashi et al, 1994), which is physiologically expressed not only in developing (Shirasawa et al, 1993; Poncet et al, 1996; Cram et al, 1999) or regenerating (Figarella-Branger et al, 1993) tissue, but also in granulocytes, pre-B-cells, keratinocytes (Redondo et al, 1998) and renal tubules (Droz et al, 1990). In neoplasia, CD24 expression has been described not only in haematologic malignancies (Pirruccello and Lang, 1990; Raife et al, 1994; Lavabre-Bertrand et al, 1994), but also in a large variety of solid tumours, for example, renal cell carcinoma (Droz et al, 1990), small cell lung cancer (Jackson et al, 1992), nasopharyngeal carcinoma (Karran et al, 1995), hepatocellular carcinoma (Huang and Hsu, 1995), bladder carcinoma (Gromova et al, 1999), glioma (Senner et al, 1999), breast cancer (Fogel et al, 1999; Yang et al, 1999; Liu and Vadgama, 2000) and ovarian cancer (Welsh et al, 2001; Kristiansen et al, 2002). CD24 is a ligand of P-selectin (Sammar et al, 1994), an adhesion receptor on activated endothelial cells and platelets, and could thus contribute to the metastasising capacities of CD24-expressing tumour cells (Aigner et al, 1995, 1997, 1998; Friederichs et al, 2000). Recently, we described CD24 as an independent prognostic marker of shortened patient survival in ovarian cancer (Kristiansen et al, 2002). To date very little is known about CD24 in NSCLC.
The aim of this study was to evaluate the status of CD24 expresssion in our set of NSCLC patients and to investigate the association of CD24-protein expression determined by immunohistochemistry with clinicopathological parameters, disease stage according to UICC and patient survival.
Materials and methods
Tumour samples
Our collective consisted of 89 patients between age 34 and 80 years (mean 62) who underwent thoracotomy for resection of NSCLC in the Department of Surgery at the Charité University Hospital from 1995 to 1997, and whose clinical follow-up data were available. No adjuvant radiotherapy or chemotherapy was applied before surgery. Table 1 summarises the clinicopathological characteristics according to TNM criteria of the UICC (Sobin and Wittekind, 1997). The histopathological diagnosis was established in every case according to the WHO guidelines (Travis, 1999). Only primary carcinomas were included in the study.
Tissue array generation
A tissue microarray (TMA) was constructed as previously described (Kristiansen et al, 2001). Briefly, suitable areas for tissue retrieval were marked on standard haematoxylin–eosin (HE) sections, punched out of the paraffin block and inserted into a recipient block. The tissue arrayer was purchased from Beecher Instruments (Woodland, USA). The punch diameter was 0.6 mm. The lung tumour array consisted of 127 tissue samples: 89 tumours and 38 punches of normal or peritumoural lung tissue. For comparison of the tissue array immunostaining with conventional slides, whole mount paraffin sections were cut from 15 cases that were incorporated in the tissue array.
Cell lines and primary cell culture
Four primary tumour cell lines of lung adenocarcinomas were established in our laboratory derived from patients with primary cancers who had surgery for NSCLC at the Charité University Hospital (D51, D54, D97, D117). These cells were grown in Leibovitz 15 media supplemented with 10% FCS and 1% glutamine. Small cell lung cancer cell lines (H526, H378, DMS-79, SHP-77) and the lung squamous cell cancer cell line H2170 were purchased from the ATCC (Rockville, MD, USA). Lung adenocarcinoma cell lines A427 and A549 were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). All cells were grown as recommended. The cell lines were centrifuged and the pellets were formalin fixed and embedded in 0.8% agarose. These agarose pellets were finally paraffinised and conventionally sectioned for immunohistochemistry.
Immunohistochemistry
The tissue array block was freshly cut (4 μm), sections were mounted on superfrost slides (Menzel-Gläser, Germany) and dewaxed with xylene, and gradually hydrated. Antigen retrieval was achieved by pressure cooking in 0.01 M citrate buffer for 5 min. The primary CD24-antibody (Ab-2, clone 24C02, Neomarkers, USA) was diluted 1 : 100 using a background reducing dilution buffer from DAKO (Germany). No other blocking agents were employed. The primary antibody was incubated at room temperature for 1 h. Detection took place by the conventional labelled-streptavidin–biotin (LSAB-kit, DAKO, Germany) method with alkaline phosphatase as the reporting enzyme according to the manufacturer's instructions. Fast-Red (Sigma-chemicals, Hamburg) served as chromogen, afterwards the slides were briefly counterstained with haematoxylin and aquaeously mounted.
The CD24 staining was independently examined by two clinical pathologists (GK, YY). The staining intensity was semiquantitatively scored from absent to strong (0, 1+, 2+, 3+) for each case. Two TMA were processed to reduce case dropout.
Statistical analysis
Fishers exact test, which is especially suited for small sets of data, was used to determine the strength of association between the investigated parameters. To compare the expression of CD24 with clinicopathological parameters, 2×2 contingency tables (e.g. CD24 score 0–1 vs 2–3 and G1& G2 vs G3) were set up and the P-values were calculated. Cumulative survival curves were calculated according to the Kaplan–Meier method, differences in survival time were assessed with the log-rank test. Multivariate survival analysis was performed on all parameters that were found to be significant on univariate analysis using the Cox regression model. P-values <0.05 were considered significant. All calculations were performed using the statistical software package SPSS, version 10.0.
Results
Immunohistochemistry
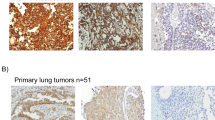
Of the 11 lung cancer cell lines, we observed a strong membraneous CD24 expression in the adenocarcinoma cell line D51 (Figure 1A). Four cell lines had a cytoplasmic staining, which was less pronounced (A427: ADC cell line; H378, DSM-79: small cell lung cancer cell lines; H2170: SCC cell line). Six cell lines were negative.
CD24 immunohistochemistry (A–F) in NSCLC. (A) Adenocarcinoma cell line with strong membraneously accentuated CD24 expression. (B) Normal lung parenchyma: membraneous expression in pneumocytes. (C) Normal lung parenchyma (on the left) with infiltrates of a poorly differentiated adenocarcinoma without CD24 expression (on the right). (D) Adenocarcinoma with moderate CD24 expression (2+). (E) Adenocarcinoma and (F) Squamous cell carcinoma with high CD24 expression (3+).
Normal lung parenchyma showed a strong membraneous staining pattern of pneumocytes (Figure 1B), whereas bronchial epithelium was mostly negative. In tumour tissue of primary lung carcinomas, CD24 immunoreactivity was observed in 68 (76%) cases with a cytoplasmic and membraneous staining pattern. The gross slides generally showed a homogenous staining of the tumours, which is a necessary condition to obtain reliable data from small tissue array punches. Specifically, on direct comparison of intensity grades of conventional whole mount sections and arrayed tissue of the same case (n=15), we found eight identical gradings, six cases were slightly different, but still fell into the same group (0–1=low-level expression vs 2–3=high-level expression), only a single case was discordant. This demonstrates that the CD24 staining of the tissue punch is representative (P=0.001, Fishers exact test).
The TMAs allowed for evaluation of 89 cases. In all, 21 cases (24%) exhibited no relevant CD24 staining (Figure 1C). In 28 cases (31%), a minimal staining was observed. A total of 21 cases (24%) showed a moderate staining intensity (Figure 1D) and 19 cases (21%) had a strong staining signal (Figure 1E, F). Taking groups three and four together, 45% of tumours showed a high level of expression of CD24 in this study ( Table 1). This was opposed to no or minimal CD24 expression (0, 1+) in the statistical analysis.
Statistical analysis
CD24 expression was significantly higher in adenocarcinomas ( Table 1, Fishers exact test, P=0.001). No correlation was found with tumour grading (P=1), tumour size (P=1), nodal status (P=0.129) or disease stage according to UICC (P=0.488).
Univariate survival analysis
Cumulative survival curves were calculated according to the Kaplan–Meier method, differences in survival were assessed with the log-rank test (Table 2). First, we analysed conventional and well-described clinicopathological parameters. Kaplan–Meier curves demonstrated a significant impact of tumour grading (P=0.001), nodal status (P=0.0001) and tumour stage (P=0.0005) on patient survival (Figures 2A–C). Tumour size (pT1 vs pT2+) was of borderline significance (P=0.083). Histological tumour type did not show a significant impact on patient survival time (P=0.566), although the mean and median survival times of squamous cell carcinomas were shorter.
For CD24 expression, we found a statistically significant difference of median survival time for tumours with low levels (0, 1+) compared to tumours with high levels (2+, 3+) of CD24 expression (Figure 2D) with 23 months vs 38 months (P=0.033).
Multivariate survival analysis
A multivariate progression analysis based on the Cox proportional hazard model was performed to test the independent value of each parameter predicting overall survival (Table 3). In this analysis, we included tumour grading (G1 and G2 vs G3), disease stage (I–II vs III–IV) and CD24 expression (0, 1+ vs 2+, 3+). All three parameters, tumour grading (P=0.011), disease stage (P=0.006) and CD24 expression (P=0.024) proved to be independent prognostic factors for shortened overall survival in NSCLC.
Discussion
In this study, 11 lung cancer cell lines and tissue of 89 NSCLC cases were immunohistologically examined for the expression of CD24 protein using a well-characterised monoclonal antibody. We found CD24 expression in 45% (five out of 11) of the cell lines: two adenocarcinoma cell lines (two out of six), two small cell lung cancer cell lines (two out of four) and one squamous cell cancer cell line (one out of one). This appears to match the data of Jackson et al, who reported CD24 expression in SCLC cell lines and in some adenocarcinoma cell lines, although unfortunately no details were given for the NSCLC cell lines investigated (Jackson et al, 1992). We found a higher incidence of CD24 expression (76% of cases) in NSCLC tissue. These different incidences of CD24 expression in cell lines and tissues might be explained in several ways. First, CD24-positive NSCLCs might be more difficult to culture as cell lines than CD24-negative tumours. Second, CD24 staining in tissue sections was often accentuated at cell–cell contacts, which are mostly missing in cell lines.
To our knowledge, this is the first study to examine the CD24 expression in a larger tumour sample of NSCLC. Methodologically, the usage of TMAs was very convenient to help processing a large set of cases. The validity of this approach was confirmed by comparison of the CD24 array staining results with matching conventional whole mount sections.
We found CD24 expression to be an independent predictor of shortened patient survival as evidenced by univariate and multivariate analyses in NSCLC. This raises the question of the biological function of CD24 in NSCLC. CD24 is expressed in a large variety of human tissues: physiologically, in developing or regenerating tissues (Figarella-Branger et al, 1993; Shirasawa et al, 1993; Poncet et al, 1996; Cram et al, 1999) and a few mature cell types such as, for example, granulocytes, keratinocytes (Redondo et al, 1998) and renal tubules (Droz et al, 1990). Pathologically, CD24 has been described in B-cell neoplasia (Pirucello and Lang, 1990; Raife et al, 1994; Lavabre-Bertrand et al, 1994), renal cell carcinoma (Droz et al, 1990), small cell lung cancer (Jackson et al, 1992), nasopharyngeal carcinoma (Karran et al, 1995), hepatocellular carcinoma (Huang and Hsu, 1995), bladder carcinoma (Gromova et al, 1999), glioma (Senner et al, 1999), breast cancer (Fogel et al, 1999; Liu and Vadgama, 2000) and ovarian cancer (Welsh et al, 2001; Kristiansen et al, 2002). This ubiquitous expression obviously excludes a diagnostic use of CD24 as a specific marker for NSCLC or any other tumour type.
CD24 has been identified as a ligand to P-selectin (Sammar et al, 1994), and it is conceivable that this function contributes to a more aggressive metastatic behaviour of CD24-positive tumour cells, as in vitro evidence suggests (Aigner et al, 1995, 1997, 1998; Friederichs et al, 2000). P-selectin is a surface molecule expressed by activated endothelial cells and platelets (McEver, 1991). It plays an important role in marginal adhesion and migration of cells under shear forces in the blood stream. Moreover, P-selectin deficiency has been linked to decreased rates of metastasis formation in mice, which underscores the relevance of this adhesion receptor in tumor progression (Kim et al, 1998). Physiologically, its primary ligand is P-Selectin Glycoprotein Ligand-1 (PSGL-1), which is expressed, for example, by neutrophils. Possibly, CD24-expressing tumour cells can spread more easily because of their capacity to either form thrombi with activated platelets or to adhere to endothelia in the bloodstream as demonstrated for CD24-expressing breast cancer cells (Aigner et al, 1997). This mechanism of metastasis does not only require expression of CD24 of the tumour, but also expression of P-selectin in platelets and endothelia of the vasculature of the target organs. Obviously, P-selectin expression on platelets and endothelia of lung cancer patients can hardly be studied comprehensively in vivo. Importantly, P-selectin-mediated binding of SCLC to endothelial cells has already been shown in vitro (Pottratz et al, 1996) and the CD24/P-selectin pathway was found to initiate lung colonisation of the human lung adenocarcinoma cell line A125 in a mouse model (Friederichs et al, 2000).
These observations are consistent with the view that CD24 is a cell surface protein that might be important for haematogenous metastasis formation, and we feel that this is the most likely explanation of the decreased survival times of strongly CD24- expressing tumours because most lung cancer patients die from systemic metastatic disease. In our study, clinicopathological parameters were only considered at the time of surgery. Thus, we did not find an association of CD24 with clinical stage since patients with haematogenous metastases are excluded from surgical therapy and are consequently not represented in our tumour sample. Also, the nonsignificant association of CD24 expression with nodal status is compatible with this interpretation, since lymphatic spread is a biologically different process, although it frequently precedes haematogenous dissemination.
This study establishes CD24 expression as an independent prognostic tumour marker in NSCLC. Our recent description of CD24 expression as an independent marker of shortened patient survival in epithelial ovarian cancer (Kristiansen et al, 2002) further underscores the importance of CD24 in metastatic disease progression of human carcinomas. Prospectively, CD24 might aid the clinician in the selection of an appropriate therapy for individual patients. In this circumstance, it is of relevance to know that the intravenous administration of CD24-specific antibodies has been sucessfully used therapeutically to treat transplantation-associated B-cell proliferative syndrome (Fischer et al, 1991; Garnier et al, 2002). This new therapeutic option might deserve consideration in NSCLC patients as well.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aigner S, Ruppert M, Hubbe M, Sammar M, Sthoeger Z, Butcher EC, Vestweber D, Altevogt P (1995) Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol 7: 1557–1565
Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K (1998) CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J, 12: 1241–1251
Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, Altevogt P (1997) CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood 89: 3385–3395
Akashi T, Shirasawa T, Hirokawa K (1994) Gene expression of CD24 core polypeptide molecule in normal rat tissues and human tumour cell lines. Virchows Arch 425: 399–406
Cram DS, McIntosh A, Oxbrow L, Johnston AM, DeAizpurua HJ (1999) Differential mRNA display analysis of two related but functionally distinct rat insulinoma (RIN) cell lines: identification of CD24 and its expression in the developing pancreas. Differentiation 64: 237–246
Droz D, Zachar D, Charbit L, Gogusev J, Chrétien Y, Iris L (1990) Expression of the human nephron differentiation molecules in renal cell carcinoma. Am J Pathol 137: 895–905
Figarella-Branger D, Moreau H, Pellissier JF, Bianco N, Rougon G (1993) CD24, a signal-transducing molecule expressed on human B lymphocytes, is a marker for human regenerating muscle. Acta Neuropathol (Berl) 86: 275–284
Fischer A, Blanche S, Le Bidois J, Bordigoni P, Garnier JL, Niaudet P, Morinet F, Le Deist F, Fischer AM, Griscelli C (1991) Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med 324: 1451–1456
Fischer GF, Majdic O, Gadd S, Knapp W (1990) Signal transduction in lymphocytic and myeloid cells via CD24, a new member of phosphoinositol-anchored membrane molecules. J Immunol 144: 638–641
Fogel M, Friederichs J, Zeller Y, Husar M, Smirnov A, Roitman L, Altevogt P, Sthoeger ZM (1999) CD24 is a marker for human breast carcinoma. Cancer Lett 143: 87–94
Friederichs J, Zeller Y, Hafezi-Moghadam A, Grone HJ, Ley K, Altevogt P (2000) The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res 60: 6714–6722
Garnier JL, Stevenson G, Blanc-Brunat N, Touraine JL, Milpied N, Leblond V, Blay JY (2002) Treatment of post-transplant lymphomas with anti-B-cell monoclonal antibodies. Recent Results Cancer Res 159: 113–122
Gromova I, Gromov P, Celis JE (1999) Identification of true differentially expressed mRNAs in a pair of human bladder transitional cell carcinomas using an improved differential display procedure. Electrophoresis 20: 241–248
Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, Smith JP (1999) Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 354: 99–105
Huang LR, Hsu HC (1995) Cloning and expression of CD24 gene in human hepatocellular carcinoma: a potential early tumour marker gene correlates with p53 mutation and tumour differentiation. Cancer Res 55: 4717–4721
Jackson D, Waibel R, Weber E, Bell J, Stahel RA (1992) CD24, a signal-transducing molecule expressed on human B cells, is a major surface antigen on small cell lung carcinomas. Cancer Res 52: 5264–5270
Jemal A, Thomas A, Murray T, Thun M (2002) Cancer Statistics, 2002. CA: Cancer J Clin 52: 23–47
Karran L, Jones M, Morley G, van Noorden S, Smith P, Lampert I, Griffin BE (1995) Expression of a B-cell marker, CD24, on nasopharyngeal carcinoma cells. Int J Cancer 60: 562–566
Kim YJ, Borsig L, Varki NM, Varki A (1998) P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA 95: 9325–9330
Kristiansen G, Denkert C, Schlüns K, Dahl E, Pilarsky C, Hauptmann S (2002) CD24 is expressed in ovarian cancer and is a new independent prognostic Marker of patient survival. Am J Pathol 161: 1215–1221
Kristiansen G, Yu Y, Petersen S, Kaufmann O, Schluns K, Dietel M, Petersen I (2001) Overexpression of c-erbB2 protein correlates with disease-stage and chromosomal gain at the c-erbB2 locus in non-small cell lung cancer. Eur J Cancer 37: 1089–1095
Lavabre-Bertrand T, Duperray C, Brunet C, Poncelet P, Exbrayat C, Bourquard P, Lavabre-Bertrand C, Brochier J, Navarro M, Janossy G (1994) Quantification of CD24 and CD45 antigens in parallel allows a precise determination of B-cell maturation stages: relevance for the study of B-cell neoplasias. Leukemia 8: 402–408
Liu W, Vadgama JV (2000) Identification and characterization of amino acid starvation-induced CD24 gene in MCF-7 in human breast cancer cells. Int J Oncol 16: 1049–1054
McEver RP (1991) GMP-140: a receptor for neutrophils and monocytes on activated platelets and endothelium. J Cell Biochem 45: 156–161
Pirruccello SJ, Lang MS (1990) Differential expression of CD24-related epitopes in mycosis fungoides/Sezary syndrome: a potential marker for circulating Sezary cells. Blood 76: 2343–2347
Pirruccello SJ, LeBien TW (1986) The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J Immunol 136: 3779–3784
Poncet C, Frances V, Gristina R, Scheiner C, Pellissier JF, Figarella-Branger D (1996) CD24, a glycosylphosphatidylinositol-anchored molecules is transiently expressed during the development of human central nervous system and is a marker of human neural cell lineage tumours. Acta Neuropathol (Berl) 91: 400–408
Pottratz ST, Hall TD, Scribner WM, Jayaram HN, Natarajan V (1996) P-selectin-mediated attachment of small cell lung carcinoma to endothelial cells. Am J Physiol 271: L918–23
Raife TJ, Lager DJ, Kemp JD, Dick FR (1994) Expression of CD24 (BA-1) predicts monocytic lineage in acute myeloid leukemia. Am J Clin Pathol 101: 296–299
Redondo P, Garcia-Foncillas J, Okroujnov I, de Felipe I, Quintanilla E (1998) CD24 expression on human keratinocytes. Exp Dermatol 7: 175–178
Sammar M, Aigner S, Hubbe M, Schirrmacher V, Schachner M, Vestweber D, Altevogt P (1994) Heat-stable antigen (CD24) as ligand for mouse P-selectin. Int Immunol 6: 1027–1036
Senner V, Sturm A, Baur I, Schrell UH, Distel L, Paulus W (1999) CD24 promotes invasion of glioma cells in vivo. J Neuropathol Exp Neurol 58: 795–802
Shirasawa T, Akashi T, Sakamoto K, Takahashi H, Maruyama N, Hirokawa K (1993) Gene expression of CD24 core peptide molecule in developing brain and developing non-neural tissues. Dev Dyn 198: 1–13
Sobin LH, Wittekind Ch (1997) TNM Classification of Malignant Tumours, 5th edn. New York: Wiley-Liss.
Travis WD (1999) WHO Histological Typing of Lung and Pleural Tumours. Geneva: WHO.
Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM (2001) Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci USA 98: 1176–1181
Yang GP, Ross DT, Kuang WW, Brown PO, Weigel RJ (1999) Combining SSH and cDNA microarrays for rapid identification of differentially expressed genes. Nucleic Acids Res 27: 1517–1523
Acknowledgements
The excellent technical assistance of Britta Beyer and Eva Polzin, and helpful comments of Marie-Christin Koll on the manuscript are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kristiansen, G., Schlüns, K., Yongwei, Y. et al. CD24 is an independent prognostic marker of survival in nonsmall cell lung cancer patients. Br J Cancer 88, 231–236 (2003). https://doi.org/10.1038/sj.bjc.6600702
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6600702
Keywords
This article is cited by
-
Nucleophosmin/B23 promotes endometrial cancer cell escape from macrophage phagocytosis by increasing CD24 expression
Journal of Molecular Medicine (2021)
-
Myeloid immunosuppression and immune checkpoints in the tumor microenvironment
Cellular & Molecular Immunology (2020)
-
Identification of CD24 as a potential diagnostic and therapeutic target for malignant pleural mesothelioma
Cell Death Discovery (2020)
-
Membranous CD24 expression as detected by the monoclonal antibody SWA11 is a prognostic marker in non-small cell lung cancer patients
BMC Clinical Pathology (2015)
-
Intracellular CD24 disrupts the ARF–NPM interaction and enables mutational and viral oncogene-mediated p53 inactivation
Nature Communications (2015)