Abstract
The aim of this study is to analyse whether immunohistochemistry (IHC) applying a broad set of markers could be used to categorise ductal carcinoma in situ (DCIS) of the breast in distinct subgroups corresponding to the recently defined molecular categories of invasive carcinoma. Immunohistochemistry of pure DCIS cases constructed in tissue arrays was performed with 16 markers (oestrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR), Bcl-2, p53, Her2, insulin-like growth factor receptor, E-cadherin, epithelial membrane antigen (EMA), CA125, keratins 5/6, 14, 19, epidermal growth factor receptor, S100, and CD31). Results in 163 cases were analysed by unsupervised hierarchical clustering. Histological classification was performed by review of whole tissue sections and identified 36 well-, 55 intermediately, and 72 poorly differentiated DCISs. Unsupervised hierarchical cluster analysis categorised DCIS into two major groups that could be further subdivided into subgroups based on the expression of six markers (ER, PR, AR, Bcl-2, p53, and Her2). In the major predominantly ER/Bcl-2-positive (luminal) group, three subgroups (AR-positive (n=33), AR-negative (n=40), and mixed (n=34)) could be identified and included 34 well-differentiated DCISs. Within the major predominantly ER/Bcl-2-negative (nonluminal) group, a Her2-positive subgroup (n=34) was characterised by 31 poorly differentiated lesions. Eight triple-negative lesions, including one positive for keratin 5/6 and two positive for p53, were encountered. Intermediately differentiated DCIS shared a comparable IHC staining pattern with well-differentiated DCIS that was distinct from poorly differentiated DCIS (P<0.001). Ductal carcinoma in situ could be categorised by IHC into two major groups and five subgroups using six markers. Morphologically, intermediately differentiated DCIS seems to have more biological similarities with well-differentiated lesions as compared to poorly differentiated lesions.
Similar content being viewed by others
Main
Breast cancer encompasses a heterogeneous group of tumours, which vary in morphology, clinical presentation, and behaviour. Traditionally, breast cancers are morphologically typed according to the World Health Organization (WHO) guidelines. The latest classification recognises at least 30 different invasive tumour types (World Health Organization Classification of Tumours, 2003). There is no consensus about the classification of the noninvasive precursor of breast carcinoma, ductal carcinoma in situ (DCIS). As in other areas of pathology, a three-tier system is most often used, based on growth pattern and cytonuclear criteria, and dividing DCIS into well-, intermediately, and poorly differentiated subtypes (Holland et al, 1994). In prospective studies, this classification has proven value in risk assessment of recurrence after breast-conserving treatment and progression into invasive carcinoma (Bijker et al, 2006). However, inter- and intraobserver variability is a problem inherent to morphologic tumour classification and grading (Sloane et al, 1998); moreover, heterogeneity within DCIS lesions is not uncommon, resulting in variation in grade (Ma et al, 2003). Perou et al (2000) suggested a categorisation of invasive breast cancers based on genetic profiles into oestrogen receptor (ER)-positive (luminal A and B) and ER-negative (nonluminal) subtypes with a further subdivision of the ER-negative types into Her2-positive and basal-like subtypes. Luminal A tumours differ from luminal B tumours by a higher expression of ER-related genes and lower expression of proliferation-associated genes. It was possible to make the same categorisation by immunohistochemistry (IHC) using markers aimed at luminal, Her2, and basal-like features (Makretsov et al, 2004). The objective of this study is to classify DCIS by marker expression to improve the current morphological classifications and gain insight into the biology underlying the heterogeneity in DCIS. Therefore, tissue microarrays were constructed from a series of pure DCISs, a large set of markers were used for IHC, and unsupervised hierarchical cluster analysis was performed to evaluate results; clustering was correlated with morphologic grade of DCIS as assessed on whole tumour slides.
Materials and methods
Tissue microarray sections were constructed taking three 0.6-mm tissue cores per case, from formalin-fixed, paraffin-embedded tumour blocks with pure DCIS of 238 patients using a tissue-arraying instrument (Beecher Instruments, Silver Spring, MD, USA). Immunohistochemistry was performed on an automated stainer after pretreatment in the autoclave in citrate buffer at pH 6.0 according to standardised protocols for the different antibodies at prescribed dilutions (see Table 1). Sixteen markers were used including ER, progesterone receptor (PR), androgen receptor (AR), Her2, Bcl-2, p53, E-cadherin, epidermal growth factor receptor, insulin-like growth factor receptor, CD31, keratin 5/6, keratin 14, keratin 19, S100, epithelial membrane antigen (EMA), and CA125. The selection of antibodies was based on recent investigations in gene signature profiles in invasive breast cancer, suitability for DCIS, and availability. Staining results were semi-quantitatively scored according to the criteria in Table 1. The higher IHC score was considered as a final score in case of a difference between tissue cores. Cutoff points are shown in Table 1 and were directed to detect luminal and nonluminal (sub)groups. All cases were classified as well-, intermediately, and poorly differentiated on the whole tumour slides according to the classification of Holland et al (1994); in case of heterogeneity, the highest grade was used for analysis. The distribution of markers and histological grade among (sub)groups was analysed using the χ2-test or Fisher's exact test. Tests were two-tailed and the significance level was taken 5%. Discriminative markers underwent unsupervised hierarchical clustering analysis with average and complete linkage (Genesis 1.5.0; IGB-TUG, Graz, Austria) to organise tissue microarray score data into meaningful structures, in accordance with the more complex method used for cDNA microarrays (Weigelt et al, 2005). The impact of the markers on hierarchical cluster group results was investigated to define a final set of markers for IHC classification. Correlation between markers was determined using the Spearman's correlation coefficient. The agreement in classification of cases based on different hierarchical clustering methods (average linkage vs complete linkage) and different IHC classifications were assessed with the κ-statistic. A κ-value of 0.41–0.6 was considerate moderate agreement, 0.61–0.8 substantial agreement, and more than 0.8 near-perfect agreement. All analyses were performed in SPSS® 11.5 for Windows (SPSS, Chicago, IL, USA).
Results
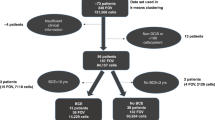
Of the 238 DCIS samples, 27 (11%) did not contain tumour and 48 (20%) had incomplete IHC data due to loss of tissue. All analyses were performed on the remaining 163 cases. Median age of these patients was 50 years (range: 28–82 years). Seventy-three per cent of the lesions were screen detected. Histological classification identified 36 (22%) well-, 55 (34%) intermediately, and 72 (44%) poorly differentiated lesions.
Marker expression in DCIS
Table 2 presents the distribution of the different markers in DCIS. Oestrogen receptor and PR were most frequently present in well- and intermediately differentiated DCIS (P<0.001), whereas Her2 expression was most frequently found in poorly differentiated DCIS (P<0.001). Also, Bcl-2 and p53 expression was different among grades: well-differentiated DCIS were often Bcl-2 positive and p53 negative compared to poorly differentiated DCIS. Thirty-three out of thirty-six well-differentiated DCISs stained positive for Bcl-2 compared to 26 out of 72 poorly differentiated DCISs (P<0.001). Further, all well-differentiated DCISs were p53 negative, while half of the poorly differentiated lesions were p53 positive (P<0.001). Moderately differentiated DCIS formed an intermediate group in expression of these markers with the exception of AR. This marker was found positive in 28 intermediately differentiated DCISs compared to 13 well-differentiated and 19 poorly differentiated DCISs (P=0.018). The remaining markers showed no statistically significant association with grade of DCIS. The E-cadherin protein could be detected in all DCISs, while markers for EGFR, CD31, keratin 14, S100, and CA125 showed negative staining results in all lesions. insulin-like growth factor receptor, keratin 19, and EMA were found positive in nearly all DCISs except seven DCISs. Five out of these seven DCISs were poorly differentiated. Three poorly differentiated DCISs showed staining for keratin 5/6.
IHC categorisation by unsupervised hierarchical analysis
Unsupervised hierarchical clustering analysis was applied to the IHC data set. On the basis of the expression of ER and Bcl-2, the clustergram in Figure 1 shows two major groups: a predominantly ER/Bcl-2-positive (luminal) and a predominantly ER/Bcl-2-negative (nonluminal) group. These two groups can be further subdivided using other marker results. The luminal group demonstrated a completely AR-positive subgroup (n=33), a completely AR-negative subgroup (n=40), and a mixed subgroup of AR-positive and -negative lesions (n=34), while the nonluminal group included a completely Her2-positive cluster (n=34). The luminal lesions included 34 (94%) well-differentiated, 46 (84%) intermediately differentiated, and 27 (38%) poorly differentiated DCISs. These poorly differentiated DCISs lesions showed markers positive for ER (all 27), Bcl-2 (n=25), PR (n=14), Her2 (n=12), AR (n=9), and p53 (n=4).
Two-dimensional unsupervised hierarchical cluster diagram (average linkage) of data consisting of 978 genes by 163 ductal carcinoma in situ samples. Clustergram of six markers and distribution of histological grade. Each column indicates a single case; each row, a single immunomarker. Green (or light grey), negative immunostaining; red (or dark grey), positive immunostaining; white, well-differentiated lesion; grey, intermediately differentiated lesion; black, poorly differentiated lesion. The dendrogram shows the relatedness of the immunoprofiles of individual cases and suggests two major groups, which are further subdivided into subgroups.
The nonluminal subgroups had 45 poorly differentiated DCISs and 11 nonpoorly differentiated lesions including nine with intermediately and two with well-differentiated DCISs. These 11 lesions showed a marker pattern that was positive for ER (n=2), Bcl-2 (n=1), Her2 (n=7), AR (n=5), and p53 (n=2). In total, eight ER-negative, PR-negative, and Her2-negative lesions, including one positive for keratin 5/6 and two positive for p53, were found. Table 3 shows the comparison of the distribution of histological grade among the identified subgroups. Intermediately differentiated DCIS significantly more often shared IHC features with well-differentiated DCIS than with poorly differentiated DCIS (P<0.001).
Reproducibility of cluster groups
For the assessment of variation in clustering results when using different hierarchical clustering methods, unsupervised hierarchical clustering by complete linkage was performed on the classification set of six markers. The concordance between designation of individual cases to one of the subgroups using average linkage vs complete linkage showed a near-perfect agreement (κ=0.876), with 16 mismatches out of 163 paired cases.
Comparison with IHC categorisation based on genetic profiles
A comparison of our results with the earlier findings from Perou et al (2000) based on genetic profiles of invasive breast carcinoma is shown in Table 4. A classification of DCIS lesions into luminal A, luminal B, Her2, and basal-like subtypes was performed on the staining results of three markers (ER, PR, and Her2) and was compared with the findings of the present study. Both classifications showed a moderate agreement (κ=0.411) mainly caused by the differentiation of the luminal types into A and B. If both the luminal types are considered as one group, the classifications demonstrated a substantial agreement (κ=0.649). Nearly complete agreement is shown for the Her2-positive and basal-like type lesions. Mixed lesions from the Perou et al (2000) classification frequently showed ER and Bcl-2 marker expression.
Discussion
The traditional histological classification of invasive breast cancer tumours has been debated by results from gene expression arrays leading to the molecular categorisation of breast cancer into luminal and nonluminal tumours (Perou et al, 2000). As invasive ductal breast cancers develop via the noninvasive precursor DCIS, these lesions may be categorised in the same way. Most similarities were found among the different stages of breast tumour progression, and it was suggested that gene expression alterations conferring the potential for invasive growth are already present in the preinvasive stadium of breast cancer (Ma et al, 2003). It has been shown that the molecular subgroups of invasive carcinoma can be distinguished using a set of IHC markers (Makretsov et al, 2004). In addition, response to treatment is subgroup dependent (Carey et al, 2007). Using IHC, our analysis focused on the identification of subgroups in pure DCIS, to improve insight into the different pathways of tumour development, and to produce a classification of DCIS based on marker expression. Sixteen markers were selected to distinguish luminal and nonluminal cell differentiation, or because of their reported value to differentiate DCIS. Unsupervised hierarchical analysis, like in the evaluation of gene expression arrays, was performed to categorise DCIS. Ten markers were either positive or negative in nearly all DCISs and therefore not useful for classification.
Oestrogen receptor, PR, AR, Her2, Bcl-2, and p53 were used for IHC classification of DCIS. Oestrogen receptor, PR, and AR were positive in 68, 46 and 37% of the patients in our series, respectively. Others found ER, PR, and AR expression in 54–73, 49–61, and 33–44%, respectively (Selim et al, 2002; Baqai and Shousha, 2003; Barnes et al, 2005; Rody et al, 2005). We further found that well- and intermediately differentiated DCISs were predominantly ER positive and PR positive, while poorly differentiated DCIS usually lacked steroid receptor expression and was correlated with Her2 overexpression. This finding became further evident by the unsupervised hierarchical clustering results that clearly divided DCIS into luminal and nonluminal lesions. The luminal-type DCIS was further divided into an AR-positive and AR-negative subtype.
Bcl-2, involved in apoptosis, was present in 64% of all DCISs, while p53 was expressed in 26% of the cases in our series. These findings are in correspondence with results from others who reported Bcl-2 and p53 expression in 76 and 24% of DCIS cases, respectively (Siziopikou et al, 1996). The Bcl-2-positive/p53-negative phenotype, which is similar to normal epithelium and benign lesions, was observed in 95 cases originating from the luminal clusters. This phenotype might reflect a more favourable group of lesions.
Androgen receptor expression was most frequently seen in intermediately and well-differentiated DCIS (P=0.018) in our series of patients. Not many studies investigated AR in DCIS. Moinfar et al (2003) reported a higher rate of AR expression in especially low-grade DCIS as opposed to high-grade DCIS, although others did not find a correlation between AR expression and grade (Selim et al, 2002). Androgen receptor-positive breast cancer patients have prolonged survival and a better response to hormonal treatment than AR-negative patients.
Within the nonluminal type, a Her2-positive/ER-negative subtype with 91% poorly differentiated DCISs could be identified. Her2 is known to be amplified and/or overexpressed in invasive breast cancer in 10–30% of cases and associated with poor outcome (Ravdin and Chamness, 1995; Tsuda, 2001). The absence of Her2 overexpression in normal ducts and atypical ductal hyperplasia, and the frequent of Her2 amplification found in DCIS suggests that Her2 alterations are an early event in the pathway of development of Her2-positive invasive carcinomas. In our study, 39% of the cases were positive for Her2. The higher frequency of Her2-positive lesions in DCIS compared with invasive breast cancer has been argued to occur due to loss of expression; however, it might indicate that in the breast cancer progression model, there may be lesions that do not frequently evolve into invasive breast cancers, including Her2-positive DCIS lesions. Moreover, the mammographic detection of poorly differentiated Her2-positive DCIS often occurs at an early stage due to the conspicuous microcalcifications.
Basal-like carcinomas have been identified in gene expression profiling studies as a subtype of invasive breast cancer. These lesions are ER negative, PR negative, and Her2 negative (triple negative). We found eight (5%) triple-negative lesions. Four of them were poorly differentiated. Bryan et al (2006) studied 66 cases of high nuclear grade DCIS to determine the frequency of the triple-negative phenotype and showed that only four cases (6%) exhibited the triple-negative phenotype. In contrast with our results, they found EGFR expressed in all four triple-negative lesions and also in a selection of nontriple-negative lesions, while we found negative staining in all lesions. In addition, they observed more frequent expression of keratins 5/6 and 14 compared with our series. This could be a result of the interobserver variability, since our cutoff point was more than 10% strong membraneous staining, while Bryan et al (2006) considered any staining as positive. Recently, (Livasy et al (2007) found 8% basal-like subtypes in a population-based series of 245 patients. Given that invasive breast cancers typically share immunophenotypic features with the DCIS lesion from which they arise, these findings corroborate the possibility that the triple-negative DCIS lesions represent a precursor lesion to invasive basal-like carcinomas. In these (medullary-like and metaplastic) carcinomas, in situ components are usually minor or absent, suggesting a rapid progression from in situ to the invasive stage. This is in keeping with the absence of basal-like in situ lesions in preventive mastectomy specimens of BRCA1 carriers, which are prone to develop basal-like tumours (Hoogerbrugge et al, 2003).
Clustering analysis showed that the well-differentiated DCIS and intermediately differentiated DCIS share IHC features among the different clusters. It seems that intermediately differentiated DCIS shows more resemblance with well-differentiated DCIS as compared with poorly differentiated DCIS. A recent study from our institute investigating classification of DCIS by gene expression profiling confirms this finding and identified luminal, Her2-, and basal-like tumours in a series of 40 DCIS lesions (Hannemann et al, 2006). A classification of DCIS by IHC might identify identical groups of luminal and nonluminal tumours, which can be further subdivided, reflecting the heterogeneous nature of DCIS. Therefore, IHC can assist in objectivation of variations in morphologic tumour classification of DCIS.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Baqai T, Shousha S (2003) Oestrogen receptor negativity as a marker for high-grade ductal carcinoma in situ of the breast. Histopathology 42: 440–447
Barnes NL, Boland GP, Davenport A, Knox WF, Bundred NJ (2005) Relationship between hormone receptor status and tumour size, grade and comedo necrosis in ductal carcinoma in situ. Br J Surg 92: 429–434
Bijker N, Meijnen P, Peterse JL, Bogaerts J, Van Hoorebeeck I, Julien JP, Gennaro M, Rouanet P, Avril A, Fentiman IS, Bartelink H, Rutgers EJ (2006) Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853 – a study by the EORTC Breast Cancer Cooperative group and EORTC Radiotherapy Group. J Clin Oncol 24: 3381–3387
Bryan BB, Schnitt SJ, Collins LC (2006) Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol 19: 617–621
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13: 2329–2334
Hannemann J, Velds A, Halfwerk JB, Kreike B, Peterse JL, van de Vijver MJ (2006) Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res 8: R61
Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, Zafrani B (1994) Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 11: 167–180
Hoogerbrugge N, Bult P, Widt-Lever LM, Beex LV, Kiemeney LV, Ligtenberg MJ, Massuger LF, Boetes C, Manders P, Brunner HG (2003) High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. J Clin Oncol 21: 41–45
Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, Tse CK, Nyante S, Millikan RC (2007) Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol 38: 197–204
Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou YX, Varnholt H, Smith B, Gadd M, Chatfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC (2003) Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA 100: 5974–5979
Makretsov NA, Huntsman DG, Nielsen TO, Yorida E, Peacock M, Cheang MC, Dunn SE, Hayes M, van de Rijn M, Bajdik C, Gilks CB (2004) Hierarchical clustering analysis of tissue microarray immunostaining data identifies prognostically significant groups of breast carcinoma. Clin Cancer Res 10: 6143–6151
Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H (2003) Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer 98: 703–711
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406: 747–752
Ravdin PM, Chamness GC (1995) The c-erbB-2 proto-oncogene as a prognostic and predictive marker in breast cancer: a paradigm for the development of other macromolecular markers – a review. Gene 159: 19–27
Rody A, Diallo R, Poremba C, Wuelfing P, Kissler S, Solbach C, Kaufmann M, Jackisch C (2005) Androgen receptor expression in ductal carcinoma in situ of the breast: not a helpful marker for classification such as estrogen receptor alpha and progesterone receptor. Appl Immunohistochem Mol Morphol 13: 25–29
Selim AG, El Ayat G, Wells CA (2002) Androgen receptor expression in ductal carcinoma in situ of the breast: relation to oestrogen and progesterone receptors. J Clin Pathol 55: 14–16
Siziopikou KP, Prioleau JE, Harris JR, Schnitt SJ (1996) bcl-2 expression in the spectrum of preinvasive breast lesions. Cancer 77: 499–506
Sloane JP, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W, Bussolati G, Coleman D, Connolly CE, Dervan P, Eusebi V, de Miguel C, Drijkoningen M, Elston CW, Faverley D, Gad A, Jacquemier J, Lacerda M, Martinez-Penuela J, Munt C, Peterse JL, Rank F, Sylvan M, Tsakraklides V, Zafrani B (1998) Consistency achieved by 23 European pathologists in categorizing ductal carcinoma in situ of the breast using five classifications. European Commission Working Group on Breast Screening Pathology. Hum Pathol 29: 1056–1062
Tsuda HH (2001) Prognostic and predictive value of c-erbB-2 (HER-2/neu) gene amplification in human breast cancer. Breast Cancer 8: 38–44
Weigelt B, Hu Z, He X, Livasy C, Carey LA, Ewend MG, Glas AM, Perou CM, van't Veer LJ (2005) Molecular portraits and 70-gene prognosis signature are preserved throughout the metastatic process of breast cancer. Cancer Res 65: 9155–9158
World Health Organization Classification of Tumours (2003) Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press
Acknowledgements
We are grateful for the technical assistance in tissue microarray analysis from Paulien Zijlstra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 17th AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, 14–18 November 2005, Philadelphia, PA, USA
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Meijnen, P., Peterse, J., Antonini, N. et al. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer 98, 137–142 (2008). https://doi.org/10.1038/sj.bjc.6604112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604112
Keywords
This article is cited by
-
Subtypes of high-grade breast ductal carcinoma in situ (DCIS): incidence and potential clinical impact
Breast Cancer Research and Treatment (2023)
-
Learning to distinguish progressive and non-progressive ductal carcinoma in situ
Nature Reviews Cancer (2022)
-
Targeted therapeutic options and future perspectives for HER2-positive breast cancer
Signal Transduction and Targeted Therapy (2019)
-
How Did We Get There? The Progression from Ductal Carcinoma In Situ to Invasive Ductal Carcinoma
Current Breast Cancer Reports (2019)
-
Intratumoral Heterogeneity in Ductal Carcinoma In Situ: Chaos and Consequence
Journal of Mammary Gland Biology and Neoplasia (2018)