Abstract
The annexins are family of calcium-regulated phospholipid-binding proteins with diverse roles in cell biology. Individual annexins have been implicated in tumour development and progression, and in this investigation a range of annexins have been studied in colorectal cancer. Annexins A1, A2, A4 and A11 were identified by comparative proteomic analysis to be overexpressed in colorectal cancer. Annexins A1, A2, A4 and A11 were further studied by immunohistochemistry with a colorectal cancer tissue microarray containing primary and metastatic colorectal cancer and also normal colon. There was significant increase in expression in annexins A1 (P=0.01), A2 (P<0.001), A4 (P<0.001) and A11 (P<0.001) in primary tumours compared with normal colon. There was increasing expression of annexins A2 (P=0.001), A4 (P=0.03) and A11 (P=0.006) with increasing tumour stage. An annexin expression profile was identified by k-means cluster analysis, and the annexin profile was associated with tumour stage (P=0.01) and also patient survival. Patients in annexin cluster group 1 (low annexin expression) had a better survival (log rank=5.33, P=0.02) than patients in cluster group 2 (high annexins A4 and A11 expression). In conclusion, this study has shown that individual annexins are present in colorectal cancer, specific annexins are overexpressed in colorectal cancer and the annexin expression profile is associated with survival.
Similar content being viewed by others
Main
The annexins are a multigene family of calcium-regulated phospholipid-binding proteins (Gerke and Moss, 2003; Gerke et al, 2005). The annexins are classified into five groups (A–E), and within each of these groups, individual annexins are identified numerically. Annexins in group A are human annexins, with group B referring to animal annexins without human orthologs, group C to fungi and moulds, group D to plants and group E to protists (Liemann and Huber, 1997; Rand, 2000; Hayes and Moss, 2004; Rescher and Gerke, 2004; Lim and Pervaiz, 2007). The characteristic annexin structural motif is a 70-amino-acid repeat, called the annexin repeat. Four annexin repeats packed into an α-helical disk are contained within the C-terminal polypeptide core (Gerke and Moss, 2003). While all annexins share this core region, the N-terminal varies widely between annexins, and it is this diversity of N-terminal amino-acid sequence that gives the individual annexins their functional differences and biological activities (Gerke and Moss, 2003; Gerke et al, 2005). There are 12 human annexin subfamilies (A1–A11 and A13) that have been found to have various intra- and extracellular roles in a range of cellular processes such as cell signalling, ion transport, cell division and apoptosis (Gerke and Moss, 2003; Gerke et al, 2005).
All annexins share an ability to bind to negatively charged phospholipid membranes in a calcium-dependent manner. This property is found within the annexin core motif where the calcium- and membrane-binding sites are located. Annexins bind to the cytosolic surface of the plasma membrane and to organelle membranes such as the Golgi apparatus. This binding can be reversed by the removal of calcium, freeing the annexin from the phospholipid membrane. However, the functional significance of their reversible membrane-binding ability remains unknown in many annexins, although in some it is thought to be important for vesicle aggregation and membrane organisation (Liemann and Huber, 1997; Rand, 2000; Rescher and Gerke, 2004; Lim and Pervaiz, 2007). Although all annexins share this binding property, there is variation in calcium sensitivity and phospholipid specificity between individual annexins. For example, within one cell there can be differences in the distribution of annexins, with annexin A1 having an endosomal localisation, A2 to be found in cytosol and A4 being associated with the plasma membrane (Liemann and Huber, 1997).
Some annexins are capable of calcium-independent binding and several have roles in vesicle aggregation. Annexins A1, A2 and A11 function in cooperation with other calcium-binding proteins to form complexes while annexins A1, A2 and A5 interact with cytoskeletal proteins. Many annexins are involved in exocytic and endocytic pathways and some have roles in ion channel regulation (Gerke and Moss, 2003). Extracellularly, annexin A1 has a role in controlling the inflammatory response while annexin A2 is present on the external surface of endothelial cells, where it may act as a receptor for ligands, including plasminogen and tissue plasminogen activator (Diaz et al, 2004; Roda et al, 2006). Extracellular annexin A5 is thought to be involved in the anticoagulation process (Rand, 2000; Hayes and Moss, 2004).
Annexins have been implicated in several disease processes, including inflammation and neoplasia (Rand, 2000). Alterations in the expression of individual annexins have been associated with tumorigenesis in several types of tumour. Loss of annexin A1 has been found to be an early event in oesophageal squamous cell carcinoma, and it may function as a tumour suppressor in the development of this type of tumour (Paweletz et al, 2000). Expression of annexin A1 has also been associated with poorly differentiated oesophageal tumours of higher tumour stage (Hu et al, 2004). Together, these studies suggest that dysregulation of annexin A1 loss is important in oesophageal tumour development and progression. Other types of cancer in which the expression of annexin A1 has been shown to be altered include pancreatic adenocarcinoma (Bai et al, 2004), where it was found to show increased expression in the majority of tumours, renal cell carcinoma (Zimmermann et al, 2007), prostate adenocarcinoma (Patton et al, 2005), breast cancer (Shen et al, 2006) and B-cell non-Hodgkin's lymphoma (Vishwanatha et al, 2004).
Other annexins have also been implicated in tumorigenesis. Overexpression of annexin A2 has been found in renal cell cancer, where it is associated with tumour stage (Zimmermann et al, 2004a), invasive breast cancer (Sharma et al, 2006) and sarcomas, including both soft tissue sarcomas (Syed et al, 2007) and osteosarcomas (Gillette et al, 2004). There is increased expression of annexin A4 in renal clear cell carcinoma (Zimmermann et al, 2004b). In prostate cancer, decreased expression of annexin A4 has been shown to correlate with worsen pathological stage (Xin et al, 2003), and loss of annexin A7 has been associated with metastatic and local recurrences of hormone refractory prostate cancer (Srivastava et al, 2001).
However, the annexins have received no significant study in colorectal cancer and in this study we used comparative proteomic analysis to identify proteins that are overexpressed in colorectal cancer, compared with morphologically normal colorectal mucosa. The annexins A1, A2, A4 and A11 were identified as four such proteins. In order to further define the roles played by these proteins in colorectal neoplasia, their expression and cellular localisation was studied by immunohistochemistry in a large series of colorectal cancers represented within a colorectal cancer tissue microarray.
Materials and methods
Proteomics
Two-dimensional gel electrophoresis and matrix-assisted laser desorption ionisation time of flight mass spectrometry (MALDI-TOF MS) on normal colon and colorectal cancer were performed as previously described (Lawrie et al, 2004; Dundas et al, 2005; Coghlin et al, 2006). Proteins were solubilised from Dukes C adenocarcinoma tissue samples and patient-matched morphologically normal colorectal mucosa (n=10 pairs of tumour and normal samples). Two-dimensional gel electrophoresis was performed using 3–10 pI immobilon strips with proteins being separated according to charge, and subsequently molecular weight. Following completion of the electrophoresis, gels were stained with Coomassie blue to visualise protein spots. The spots were excised from the gels and peptide mass mapping was performed using a PerSeptive Biosystems Voyager-DE STR mass spectrometer. The masses of the tryptic fragments were determined and entered into the MS-Fit database-searching program (http://prospector.ucsf.edu/ucsfhtml14.0/msfit.htm). The database was restricted to searching for human proteins, but no restrictions were placed on either molecular weight or isoelectric point. To ensure that proteins were accurately identified, a significant difference in statistical score (MOWSE score) between proteins ranked first and second in the results had to be obtained.
Antibodies
A polyclonal antibody to annexin A4 was produced in our own laboratory. An immunising peptide was designed, which took into consideration both antigenicity and hydrophilicity. Furthermore, a blast search was used to determine that the peptide was unique to annexin A4 (SVLAYRNTA corresponding to amino acids 39–47 of annexin A4), thus ensuring no crossreactivity with other proteins. The annexin IV peptide conjugated to ovalbumin was used to immunise rabbits subsequently followed with four boosters, with a protocol we have previously used for the development of other antibodies (Kumarakulasingham et al, 2005). Nine weeks after the initial booster, rabbits were bled and serum was tested by ELISA using the peptide as an immunogen. The sera that showed the highest antibody titre were also tested by immunoblotting using whole-cell lysates. Immunoblotting showed that the antibody recognised a protein at the expected molecular size for annexin A4.
Monoclonal antibodies to annexins A1, A2, A7 and A11 were bought from BD Biosciences (Oxford, UK).
Tumour samples and tissue microarray construction
All cases were selected from the Aberdeen colorectal tumour bank. In total, 268 patients were involved in this study; in each case, a diagnosis of primary colorectal cancer had been made, and the patients had undergone elective surgery for primary colorectal cancer, in Aberdeen, between 1994 and 2003. The Aberdeen colorectal tumour bank is linked to a comprehensive set of clinicopathological data, including age, gender, site of primary tumour, degree of tumour differentiation and tumour stage. The data for the patients included in this study are detailed in Table 1. Complete follow-up ranging from 0 to 144 months was available for all patients, and the mean patient survival was 91 months. At the time of censoring the data, there had been 106 (39.6%) deaths in the patient group. The tumour samples were submitted to the Department of Pathology, University of Aberdeen for diagnosis. The tumour excision specimens were fixed in formalin, representative blocks were embedded in wax and sections were stained with haematoxylin and eosin. Permission for this study was obtained from the Grampian Research Ethics Committee.
A colorectal cancer tissue microarray was constructed as described (Dundas et al, 2005; Kumarakulasingham et al, 2005). The tumours within the array were representative of the distribution of anatomical locations and the Dukes stages found in colorectal cancers within this population. The tissue microarray contained primary colorectal cancer (Dukes A=53, Dukes B=104 and Dukes C=111). In addition, it contained lymph node metastases and morphologically normal colonic mucosal samples. The lymph node metastases were from the corresponding Dukes C cases (n=111). Each normal sample (n=52) was acquired from at least 10 cm distant from the tumour as previously described (Kumarakulasingham et al, 2005). Using a steel Menghini needle, a representative 1.6 mm core of tissue was taken from each donor block and arrayed into the recipient wax block. In order to check the histopathological diagnosis and the adequacy of tissue sampling, a section from each microarray was stained with haematoxylin and eosin and examined by light microscopy.
Immunohistochemistry
Annexin immunohistochemistry was carried out using a Dako autostainer (Dako, Ely, UK) as previously described (Dundas et al, 2005; Coghlin et al, 2006). Sections (4 μm) of the tissue microarray were dewaxed, rehydrated and an antigen retrieval step performed when required. The antigen retrieval step consisted of microwaving the sections in 0.01 M citrate buffer at pH 6.0 for 20 min in an 800 W microwave oven operated at full power. The sections were then allowed to cool to room temperature. Primary antibody appropriately diluted (Table 2) in antibody diluent (Dako) was applied for 60 min at room temperature, washed with buffer (Dako) followed by peroxidase blocking for 5 min (Dako), followed by a single 2-min buffer wash. Prediluted peroxidase polymer-labelled goat anti-mouse/rabbit secondary antibody (Envision™, Dako) was applied for 30 min at room temperature, followed by further washing with buffer to remove unbound antibody. Sites of peroxidase activity were then demonstrated with diaminobenzidine as the chromogen applied for three successive 5 min periods. Finally, sections were washed in water, lightly counterstained with haematoxylin, dehydrated and mounted. Omitting the primary antibody from the immunohistochemical procedure and replacing it with antibody diluent or non-immune rabbit serum acted as negative controls.
The sections were evaluated by light microscopic examination, and cellular localisation and intensity (negative=0, weak=1, moderate=2, strong=3) of immunostaining in each section were assessed by two observers (RD and GIM).
Statistical analysis
Comparison of expression of individual annexins in normal colon, colon cancer and lymph node metastasis was performed with the Mann–Whitney U-test. The chi-square (χ2) test was used to compare annexin expression with tumour stage while the annexin expression profile was determined by k-means cluster analysis. The relationship of patient survival and annexin expression was determined using the method of Kaplan–Meier and the log-rank test. Cox-multivariate analysis was used to determine the relative significance of individual clinicopathological factors, annexin expression and patient survival. All the statistical analyses were performed using SPSS v15 for Windows XP™ (SPSS UK Ltd, Woking, UK).
Results
Proteomics
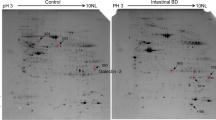
Comparative proteomic analysis using two-dimensional gel electrophoresis identified certain protein spots that were represented in the colorectal cancer samples but not in the normal colorectal mucosal samples (Figure 1). Protein spots of interest were digested and the masses of the tryptic fragments were determined using MALDI-TOF MS. These masses were entered into MS-Fit, which identified annexins A1, A2, A4 and A11 with a high degree of significance. The MOWSE score (a measure of the identity of the protein) for annexin A1 was 1.68e+003, annexin A2 was 6.53e+005, annexin A4 was 1.52e+008 and annexin A11 was 6.67e+005.
Immunohistochemistry
Primary colorectal cancer
The annexins with the exception of annexin A7 showed increased immunostaining in primary tumours in comparison to normal colon (Figures 2 and 3). Weak staining was seen for all annexins varying from 0.4 to 25.9% of tumours. Moderate and strong tumour cell staining was seen in annexins A1, A2, A4 and A11. More tumours showed strong staining for A1, A2 and A4 than showed moderate staining. The highest percentage of strong staining was seen in annexin A4, with 64.9% of tumours showing strong staining. However, for annexin A11, moderate staining was observed in 33.3% of tumours compared to 31.1% of tumours that showed strong staining (Figures 2 and 3). There was significant increase in expression in annexins A1 (P=0.01), A2 (P<0.001), A4 (P<0.001) and A11 (P<0.001) in primary tumours compared with normal colon (Figure 3). There was increased expression of annexins A2, A4 and A11 with increasing tumour stage (Table 3).
Lymph node metastasis
The annexins except annexin A7 showed immunoreactivity in lymph node metastasis. Annexins A2 and A11 showed the greatest percentage of weak staining at 20.7 and 29%, respectively, with less tumours showing moderate and strong staining for these annexins. As in normal and primary tumours, annexin A4 showed the greatest percentage of strong staining at 62.3% of tumours. Comparing the expression of annexins in lymph node metastasis with the corresponding primary tumours showed that there was a significant decrease in expression of annexin A11 (P=0.01) in lymph node metastasis compared with corresponding primary colorectal cancers (Figure 4).
Annexin expression profile and clinicopathological factors
To further dissect the role of annexin expression in colorectal cancer, the annexin expression profile was determined. To identify the relationship of the overall annexin profile within tumours, k-means cluster analysis was performed and this identified four clusters or groups with distinct annexin profiles (Table 4). The annexin profile was associated with Dukes stage (χ2=16.76, P=0.01). The annexin expression profile was also associated with survival. Patients in annexin cluster group 1 (low annexin expression) had a better survival (log rank=5.33, P=0.02; Figure 5) than patients in cluster group 2 (high annexin A4 and A11 expression). The mean survival in group 1 was 96 months (95% CI: 80–113 months) and in group 2 was 72 months (95% CI: 62–82 months). However, there was no relationship between the expression of individual annexins and patient survival and also the annexin profile was not an independent marker of prognosis following multivariate analysis.
Discussion
The annexins are a multigene family of calcium-dependent phospholipid-binding proteins (Gerke and Moss, 2003; Hayes and Moss, 2004; Gerke et al, 2005). There are 12 human annexins each of which shows a cell- and tissue-type-specific pattern of expression. Some of the annexins have been well characterised while less is known about the biology of some of the other annexins. Functions of the annexins include vesicle aggregation and ion channel regulation as well as roles in cell cycle regulation, cell signalling, cell differentiation and as extracellular receptors. Several of the annexins have been linked to the pathogenesis of a variety of disease processes, including the development and progression of several different types of cancer, and from these studies there appears to be tumour-type-specific alterations in the expression of individual annexins (Paweletz et al, 2000; Gerke and Moss, 2003; Xin et al, 2003; Bai et al, 2004; Gillette et al, 2004; Hayes and Moss, 2004; Hu et al, 2004; Vishwanatha et al, 2004; Patton et al, 2005; Sharma et al, 2006; Shen et al, 2006; Zimmermann et al, 2004a, 2004b, 2007; Syed et al, 2007). However, the annexin profile has not previously been investigated in individual tumour types. Furthermore, while there has been a range of studies of individual annexin expression in specific types of primary tumours, there have been no significant previous studies of annexin expression in metastasis. In addition, more recently individual annexins have been proposed as putative tumour biomarkers and potential therapeutic targets in cancer (Oh et al, 2004; Falsey et al, 2006; Wozny et al, 2007).
The expression and cellular localisation of annexins have received very little previous study in colorectal cancer, with only annexin A2 having been investigated (Emoto et al, 2001). In this study, comparative proteomic analysis utilising two-dimensional gel electrophoresis and mass spectrometry identified four annexins, namely A1, A2, A4 and A11 to be overexpressed in colorectal cancer when compared with normal colon. The expression profile of these annexins was then validated on a large cohort of well-characterised colorectal cancer samples, and the immunohistochemical component of the study was also extended to include a further annexin, annexin A7, as it has also been implicated in tumorigenesis (Srivastava et al, 2001). The relationship of annexin expression with clinicopathological parameters, in particular, tumour stage and overall patient survival was also investigated.
Annexins A1, A2, A4 and A11 were found to be overexpressed in primary colorectal cancer and expression of the annexins A2, A4 and A11 all increased significantly with advancing tumour stage. These findings suggest that annexins A2, A4 and A11 have an important role in the progression of colorectal cancer. In support of these findings, several studies have implicated individual annexins in tumorigenesis. Annexin A1 has been reported to show altered expression in a variety of different cancers, including oesophageal cancer (Paweletz et al, 2000; Hu et al, 2004; Wang et al, 2006), pancreatic cancer (Bai et al, 2004) and hairy cell leukaemia (Falini et al, 2004). In oesophageal adenocarcinoma, the tumour cell expression of annexin A1 has been associated with poor prognosis (Wang et al, 2006). Furthermore, annexin A1 has been found to be overexpressed in immortalised colorectal cell lines (Guzman-Aranguez et al, 2005). This annexin has also a potential role in tumour invasion and metastasis, as inhibition of annexin A1 using siRNA resulted in a significant reduction of cell invasion using an in vitro assay on an immortalised colorectal cancer cell line (Babbin et al, 2006).
Annexin A2 shows increased expression in several type of cancer, including renal cell cancer (Zimmermann et al, 2004a), breast cancer (Sharma et al, 2006) and sarcomas (Gillette et al, 2004; Syed et al, 2007), and there are several possible mechanisms by which annexin A2 may be involved in tumour progression. Annexin A2 interacts with tissue-type plasminogen activator and disruption of this interaction resulted in decreased tumour cell invasion (Rand, 2000; Diaz et al, 2004; Sharma et al, 2006). Annexin A2 is also known to form a complex with cathepsin B that can initiate proteolytic cascades and degrade extracellular matrix proteins. These functions may enhance tumour cell detachment, invasion and motility and thus promote tumour invasion and metastasis (Mai et al, 2000). Cell-surface annexin A2 also acts as a receptor for tenascin C, a key extracellular matrix protein involved in epithelial–stromal interactions, and increased annexin A2 expression is associated with progression in pancreatic neoplasia from pancreatic intraepithelial neoplasia through to invasive pancreatic carcinoma (Esposito et al, 2006). Recently, it has also been shown that the production of matrix metalloproteinase 1, a key enzyme promoting colorectal cancer invasion (Murray et al, 1996), can be mediated by annexin A2. Inhibition of annexin A2 was associated with loss of production of this matrix-degrading enzyme (Zhang et al, 2007).
Renal clear cell carcinoma also shows overexpression of annexin A4 and this seems to be related to the metastatic potential of this type of tumour (Zimmermann et al, 2004b). Annexin A4 had a distinct subcellular localisation in tumour cells and this was linked to loss of cell-to-cell adhesion and increased tumour cell dissemination (Zimmermann et al, 2004b). Additionally, it has been demonstrated that overexpressed annexin A4 promotes cell migration in a model tumour system (Zimmermann et al, 2004b), which correlates with our observation that annexin A4 expression increased as tumour stage progressed, such findings are indicative that annexin A4 is implicated in tumour spread. Annexin A4 is known to form complexes with protein kinase C, and there are 10 isoforms of protein kinase C that have roles in cancer progression (metastasis) and some of these isoforms have been shown to be overexpressed in colorectal cancer (Gokmen-Polar et al, 2001). It could be through association with protein kinase C isoforms that annexin A4 has an effect on the pathogenesis of colorectal cancer. Annexin A4 has also been shown to be overexpressed in a paclitaxel-resistant cell line and, moreover, overexpression of annexin A4 in this cell line resulted in a four-fold increase in paclitaxel resistance also indicating a role for annexins in anticancer drug resistance (Han et al, 2000).
Annexin A11 was overexpressed in colorectal cancer and increased expression correlated with more advanced tumour stage. Annexin A11 is implicated as being involved in cell growth (Farnaes and Ditzel, 2003) and a reduction in annexin A11 expression using RNAi stops cell division (Tomas et al, 2004). However, annexin A11 expression was decreased in metastasis, suggesting further dysregulation of this protein with tumour progression and possibly indicating that the tumour microenvironment plays a role in regulating annexin A11, although the specific mechanisms regulating this annexin remain to be elucidated.
Annexin A7 expression was not detected in either normal colon or colorectal cancer, whereas annexin A7 has been proposed as a putative tumour suppressor gene in prostate cancer (Srivastava et al, 2001) and that high expression of annexin A7 is associated with poor prognosis in breast cancer (Srivastava et al, 2004), thus providing further evidence that there is tumour-type-specific regulation and expression of individual annexins.
In conclusion, this study has shown that annexins A1, A2, A4 and A11 are significantly overexpressed in colorectal cancer and that the overexpression of annexins A2, A4 and A11 showed a significant correlation with increasing tumour stage. The overall expression profile of annexins was associated with survival in colorectal cancer, indicating collectively that annexin expression may contribute to outcome and this would be consistent with the putative roles of annexins in some of the cellular processes that led to tumour invasion. These annexins may also represent tumour biomarkers and potential therapeutic targets (Oh et al, 2004; Falsey et al, 2006; Wozny et al, 2007).
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Peretti M, Nusrat A (2006) Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem 28: 19588–19599
Bai XF, Ni XG, Zhao P, Liu SM, Wang HX, Guo B, Zhou LP, Liu F, Zhang JS, Wang K, Xie YQ, Shao YF, Zhao XH (2004) Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol 10: 1466–1470
Coghlin C, Carpenter B, Dundas SR, Lawrie LC, Telfer C, Murray GI (2006) Characterisation and over-expression of chaperonin T-complex proteins in colorectal cancer. J Pathol 210: 351–357
Diaz VM, Hurtado M, Thomson TM, Reventos J, Paciucci R (2004) Specific interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro. Gut 53: 993–1000
Dundas SR, Lawrie LC, Rooney PH, Murray GI (2005) Mortalin is overexpressed by colorectal cancer and correlates with poor survival. J Pathol 205: 74–81
Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, Kamada K, Naito A, Hirao S, Nakajima Y (2001) Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer 92: 1419–1426
Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess H, Schirmacher P (2006) Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol 208: 673–685
Falini B, Tiacci E, Liso A, Basso K, Sabattini E, Pacini R, Foa R, Pulsoni A, Dalla Favera R, Pileri S (2004) Simple diagnostic assay for hairy cell leukaemia by immunocytochemical detection of annexin A1 (ANXA1). Lancet 363: 1869–1870
Falsey RR, Marron MT, Gunaherath GM, Shirahatti N, Mahadevan D, Gunatilaka AA, Whitesell L (2006) Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat Chem Biol 2: 33–38
Farnaes L, Ditzel HJ (2003) Dissecting the cellular functions of annexin XI using recombinant human annexin XI-specific autoantibodies cloned by phage display. J Biol Chem 278: 33120–33126
Gerke V, Creutz CE, Moss SE (2005) Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 6: 449–461
Gerke V, Moss SE (2003) Annexins: from structure to function. Physiol Rev 82: 331–371
Gillette JM, Chan DC, Nielsen-Preiss SM (2004) Annexin 2 expression is reduced in human osteosarcoma metastases. J Cell Biochem 92: 820–832
Gokmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP (2001) Elevated protein kinase C betaII is an early promotive event in colon carcinogenesis. Cancer Res 61: 1375–1381
Guzman-Aranguez A, Olmo N, Turnay J, Lecona E, Perez-Ramos P, Lopez SI, Lizarbe MA (2005) Differentiation of human colon adenocarcinoma cells alters the expression and intracellular localization of annexins A1, A2, and A5. J Cell Biochem 94: 178–193
Han EK, Tahir SK, Cherian SP, Collins N, Ng SC (2000) Modulation of paclitaxel resistance by annexin IV in human cancer cell lines. Br J Cancer 83: 83–88
Hayes MJ, Moss SE (2004) Annexins and disease. Biochem Biophys Res Commun 322: 1166–1170
Hu N, Flaig MJ, Su H, Shou JZ, Roth MJ, Li WJ, Wang C, Goldstein AM, Li G, Emmert-Buck MR, Taylor PR (2004) Comprehensive characterization of annexin I alterations in esophageal squamous cell carcinoma. Clin Cancer Res 10: 6013–6022
Kumarakulasingham M, Rooney PH, Dundas SR, Telfer C, Melvin WT, Curran S, Murray GI (2005) Cytochrome P450 profile of colorectal cancer: identification of markers of prognosis. Clin Cancer Res 11: 3759–3764
Lawrie LC, Dundas SR, Curran S, Murray GI (2004) Liver fatty acid binding protein expression in colorectal neoplasia. Br J Cancer 90: 1955–1960
Liemann S, Huber R (1997) Three-dimensional structure of annexins. Cell Mol Life Sci 53: 516–521
Lim LH, Pervaiz S (2007) Annexin 1: the new face of an old molecule. FASEB J 21: 968–975
Mai J, Waisman DM, Sloane BF (2000) Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim Biophys Acta 1477: 215–230
Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE (1996) Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med 2: 461–462
Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE (2004) Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 429: 629–635
Patton KT, Chen HM, Joseph L, Yang XJ (2005) Decreased annexin I expression in prostatic adenocarcinoma and in high-grade prostatic intraepithelial neoplasia. Histopathology 47: 597–601
Paweletz CP, Ornstein DK, Roth MJ, Bichsel VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH, Herring J, Wang QH, Hu N, Linehan WM, Taylor PR, Liotta LA, Emmert-Buck MR, Petricoin EF (2000) Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res 60: 6293–6297
Rand JH (2000) The annexinopathies: a new category of diseases. Biochim Biophys Acta 1498: 169–173
Rescher U, Gerke V (2004) Annexins – unique membrane binding proteins with diverse functions. J Cell Sci 117: 2631–2639
Roda O, Chiva C, Espuna G, Gabius HJ, Real FX, Navarro P, Andreu D (2006) A proteomic approach to the identification of new tPA receptors in pancreatic cancer cells. Proteomics 6 (Suppl 1): S36–S41
Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC (2006) Angiogenesis-associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol 81: 146–156
Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, Chia D, Seligson D, Chang HR, Goodglick L (2006) Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol 37: 1583–1591
Srivastava M, Bubendorf L, Raffeld M, Bucher C, Torhorst J, Sauter G, Olsen C, Kallioniemi OP, Eidelman O, Pollard HB (2004) Prognostic impact of ANX7-GTPase in metastatic and HER2-negative breast cancer patients. Clin Cancer Res 10: 2344–2350
Srivastava M, Bubendorf L, Srikantan V, Fossom L, Nolan L, Glasman M, Leighton X, Fehrle W, Pittaluga S, Raffeld M, Koivisto P, Willi N, Gasser TC, Kononen J, Sauter G, Kallioniemi OP, Srivastava S, Pollard HB (2001) ANX7, a candidate tumor suppressor gene for prostate cancer. Proc Natl Acad Sci USA 98: 4575–4580
Syed SP, Martin AM, Haupt HM, Arenas-Elliot CP, Brooks JJ (2007) Angiostatin receptor annexin II in vascular tumors including angiosarcoma. Hum Pathol 38: 508–513
Tomas A, Futter C, Moss SE (2004) Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J Cell Biol 165: 813–822
Vishwanatha JK, Salazar E, Gopalakrishnan VK (2004) Absence of annexin I expression in B-cell non-Hodgkin's lymphomas and cell lines. BMC Cancer 4: 8
Wang KL, Wu TT, Resetkova E, Wang H, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton SR, Albarracin CT (2006) Expression of annexin A1 in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Clin Cancer Res 12: 4598–4604
Wozny W, Schroer K, Schwall GP, Poznanovic S, Stegmann W, Dietz K, Rogatsch H, Schaefer G, Huebl H, Klocker H, Schrattenholz A, Cahill MA (2007) Differential radioactive quantification of protein abundance ratios between benign and malignant prostate tissues: cancer association of annexin A3. Proteomics 7: 313–322
Xin W, Rhodes DR, Ingold C, Chinnaiyan AM, Rubin MA (2003) Dysregulation of the annexin family protein family is associated with prostate cancer progression. Am J Pathol 162: 255–261
Zimmermann U, Woenckhaus C, Pietschmann S, Junker H, Maile S, Schultz K, Protzel C, Giebel J (2004a) Expression of annexin II in conventional renal cell carcinoma is correlated with Fuhrman grade and clinical outcome. Virchows Arch 445: 368–374
Zimmermann U, Balabanov S, Giebel J, Teller S, Junker H, Schmoll D, Protzel C, Scharf C, Kleist B, Walther R (2004b) Increased expression and altered location of annexin IV in renal clear cell carcinoma: a possible role in tumour dissemination. Cancer Lett 209: 111–118
Zimmermann U, Woenckhaus C, Teller S, Venz S, Langheinrich M, Protzel C, Maile S, Junker H, Giebel J (2007) Expression of annexin AI in conventional renal cell carcinoma (CRCC) correlates with tumour stage, Fuhrman grade, amount of eosinophilic cells and clinical outcome. Histol Histopathol 22: 527–534
Zhang Y, Zhou ZH, Bugge TH, Wahl LM (2007) Urokinase-type plasminogen activator stimulation of monocyte matrix metalloproteinase-1 production is mediated by plasmin-dependent signaling through annexin A2 and inhibited by inactive plasmin. J Immunol 179: 3297–3304
Acknowledgements
This research was supported by grants from Knowledge Transfer Partnership, Scottish Hospital Endowments Research Trust and the University of Aberdeen Development Trust. The technical assistance of Ms Joan Aitken and Mrs Nicky Fyfe is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
GIM and CT are named inventors on a patent application made by the University of Aberdeen and Auvation Ltd to exploit the overexpression in colorectal cancer of proteins, including annexin A4, as diagnostic markers and therapeutic targets.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Duncan, R., Carpenter, B., Main, L. et al. Characterisation and protein expression profiling of annexins in colorectal cancer. Br J Cancer 98, 426–433 (2008). https://doi.org/10.1038/sj.bjc.6604128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604128
Keywords
This article is cited by
-
Molecular analysis of Annexin expression in cancer
BMC Cancer (2022)
-
Annexin A1 promotes the progression of bladder cancer via regulating EGFR signaling pathway
Cancer Cell International (2022)
-
Plasma membrane integrity in health and disease: significance and therapeutic potential
Cell Discovery (2021)
-
Short-term transcriptomic response to plasma membrane injury
Scientific Reports (2021)
-
Comparative molecular cell-of-origin classification of diffuse large B-cell lymphoma based on liquid and tissue biopsies
Translational Medicine Communications (2020)