Abstract
In addition to the direct targeting effects on HER2-positive cells, trastuzumab may have a therapeutic role modulating the activity of the cellular immune system in patients with breast cancer. To investigate this further, the balance of T-regulatory (Treg), Th17, natural killer (NK) and NK T (NKT) cells before, during and after trastuzumab therapy was investigated. Sequential frequencies of circulating Treg cells, Th17 cells, NK and NKT cells were measured in peripheral blood of breast cancer patients and normal controls throughout therapy. Individuals with breast cancer had significantly higher Treg frequencies of peripheral blood compared with healthy controls (9.2 or 8.6 vs 6%; P<0.05), and no significant differences in Treg frequencies were observed between HER2-positive and HER2-negative individuals. The number of Th17 cells was lowest in HER2-positive patients compared with both healthy controls and HER2-negative patients (0.31 vs 0.75% or 0.84%; P=0.01). There appeared to be an inverse relationship between Treg and Th17 frequencies in metastatic breast cancer (MBC) with Treg levels significantly reduced during treatment with trastuzumab (P=0.04), whereas Th17 frequencies were concomitantly increased (P=0.04). This study supports earlier data that Treg cells are present at higher frequencies in breast cancer patients compared with healthy individuals. For the first time, we show that HER2-positive individuals with breast carcinomas have reduced numbers of circulating Th17 cells, which appear, in turn to have an inverse relationship with Treg frequency in MBC. The change in balance of the Treg : Th17 ratio appears to characterise the cancer state, and furthermore, is disrupted by trastuzumab therapy.
Similar content being viewed by others
Main
Several studies have shown that higher numbers of T-regulatory (Treg) cells are associated with progression in a variety of malignancies, and Treg numbers appear increased in individuals with a variety of solid tumours (lung, breast and pancreas) and haematological malignancies, and this is reflected in the peripheral blood (PB) of cancer patients (Woo et al, 2001; Liyanage et al, 2002; Diederichsen et al, 2003; Javia and Rosenberg, 2003; Wolf et al, 2003; Marshall et al, 2004; Galon et al, 2006; Nedergaard et al, 2007). The Treg subgroup of immune cells suppresses the activity of effector cells, including CD4+ and CD8+ cytotoxic T cells, natural killer (NK) cells, dendritic cells, NK T (NKT) cells and B cells (Murakami et al, 2002; Trzonkowski et al, 2004). The outcome of this activity appears to promote the survival of cancer cells by affording protection from both the innate and adaptive immune systems.
Th17 cells have been defined as an immune subset of CD4+ lymphocytes, characterised by their production of the cytokines IL6, IL17 and TNF-α and expression of the transcription factor, RORγT. They have been implicated in inflammation and autoimmune disease, but little is known about their prevalence and function in human cancer. Initial reports have identified Th17s within ovarian tumour (Kryczek et al, 2007; Miyahara et al, 2008), but their precise role in this setting remains unclear.
The differentiation of Th17 cells is promoted by IL-23, an inducer of experimental autoimmune encephalitis (Langrish et al, 2005; McKenzie et al, 2006), and it is thought that TGF-β plays a key role in determining whether CD4+ lymphocytes become Treg or Th17 cells in vitro and possibly in vivo (Veldhoen et al, 2006; Zhou et al, 2008). The balance of Treg cells and Th17s may influence the immune response against cancer, with a Treg bias favouring tumour immune escape.
There are increasing data that the activity of regulatory cells controlling the cellular immune system may have importance in the clinical outcomes of cancer therapies (Bamias et al, 2008). Some chemotherapeutic agents, such as cyclophosphamide, have been shown to lead to reduced numbers of functional Treg cells (Beyer and Schultze, 2006). Conversely, those treated with high-dose interleukin-2 (IL-2) for melanoma and renal cell carcinoma show expansion of these cells (Ahmadzadeh and Rosenberg, 2006).
In breast cancer, it is unclear whether trastuzumab, an IgG1 monoclonal antibody, has direct effects on Treg immune subsets. Several studies have shown an increased prevalence of circulating Treg cells in breast cancer patients compared with normal controls (Liyanage et al, 2002; Perez et al, 2007), and the presence of Treg cells is likely to correlate to cancer progression. However, the effect of breast cancer therapies, such as trastuzumab, upon Treg cells and other immune subsets remains unknown. Here, we have examined the numbers of Treg cells, Th17, NK and NKT cells in Her-2/neu (HER2)-positive (early and metastatic breast cancer (MBC)) and HER2-negative breast cancer patients, with the aim of further elucidation of the role of Treg and Th17 cells, and their balance, in different patient populations with breast cancer over time.
PATIENTS AND METHODS
Patients
Between September 2007 and January 2009, patients with HER2-positive breast cancer were recruited before therapy with trastuzumab. Individuals with negative HER2 and healthy controls were also recruited. The study obtained appropriate ethical approval in accordance with the Declaration of Helsinki. Peripheral blood was collected before and during therapy into sodium heparin tubes, and PB mononuclear cells (PBMCs) were isolated by density centrifugation, and frozen in liquid nitrogen until analysis.
Patients receiving adjuvant trastuzumab had completed cytotoxic chemotherapy ⩾3 weeks before the baseline blood sample, and then went on to receive trastuzumab monotherapy. The majority of individuals whom we studied receiving trastuzumab for metastatic disease also received monotherapy, but a minority were treated in combination with docetaxel or vinorelbine. Samples were taken before the beginning of the treatment, during the treatment (one or more time points), and for early breast cancer (EBC) patients, samples were also taken following the completion of the course of adjuvant trastuzumab. In addition, samples from seven HER2-negative patients and four healthy donors (all age-matched females) were also obtained. As we treat patients with metastatic cancer with trastuzumab beyond progression, we did not obtain ‘metastatic samples’ post-treatment for this study.
Cell staining
Before intracellular staining, PBMCs were thawed and restimulated for 4 h with 50 ng ml−1 phorbol 12-myristate 13-acetate (PMA) and 100 ng ml−1 ionomycin in the presence of (10 μg ml−1) protein transport inhibitor Brefeldin A (Sigma, Poole, UK). Cells were surface stained with combinations of CD4-APC (clone RPA-T4), CD3-PE (UCHT1), CD25-FITC (BC96), CD45RO-FITC (UCHL1), CD56-FITC (MEM188), GITR-PE (ebioAITR) and CTLA-4-PE (14D3; all antibodies from eBioscience, San Diego, CA, USA). Cells were fixed and permeabilised with the FoxP3 fix/perm kit (eBioscience), and intracellularly stained with PE-conjugated antibodies against FoxP3 and IL-17A (clones PCH101 and eBio64DEC17, respectively, from eBioscience). Isotype control antibodies were used to determine the background fluorescence.
Flow cytometry analysis
Peripheral blood mononuclear cells were analysed on a FACScalibur flow cytometer equipped with CellQuest software (BD Biosciences, San Jose, CA, USA), and data were analysed using FlowJo v. 7 (Treestar Inc., Ashland, OR, USA). Regulatory T cells were defined as CD4+FoxP3+CD25+, and the expression of GITR and CTLA were also measured. Th17 cells were defined as CD4+IL17A+; NK cells were defined as CD3-CD56+, whereas NKT cells were counted as CD3+CD56+ cells.
Statistics
All statistical comparisons between sample groups were carried out using the non-parametric Mann–Whitney U-test with P-values <0.05 considered significant. For patients who died, we used the last observation carried forward for statistical analyses.
RESULTS
Breast cancer patients have increased numbers of Treg cells
A total of 27 patients with HER2-positive breast cancer were recruited. All of these individuals were being treated with trastuzumab, either in the adjuvant setting for EBC (n=14; mean age 53 years, range: 33–64) or for MBC (n=13; mean age 58, range 32–79). During the course of this study, two HER2-positive patients died.
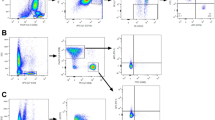
Initially, a comparison of cell frequencies between HER2-positive and HER2-negative breast cancer patients was undertaken. Regulatory T cells were characterised as CD4+FoxP3+; in addition, the expression of CD25, GITR and CTLA-4 among this population was also confirmed (Figure 1A). The absolute numbers and percentage of Treg cells in the PB of healthy individuals and breast cancer patients was determined, and our data show that individuals with breast cancer have a significantly higher Treg frequency in the PB compared with healthy controls; Treg frequencies were >8% of CD4+ cells in breast cancer, compared with 6% of CD4+ cells in healthy controls (P<0.05). The frequency of Treg cells precisely reflected the absolute numbers of Treg cells observed (as measured as the number of Treg cells per million PBMCs counted; Supplementary data figure).
Treg frequency is increased in breast cancer patients compared with healthy individuals. (A) A representative Treg FACS plot showing CD4+FoxP3+ cells. By gating on the CD4+FoxP3+ cells, it was shown that they were also CD25 expressing (shown in histogram; key to histogram: isotype control (dotted grey line), CD4+FoxP3- cells (grey line), CD4+FoxP3+ cells (black line)). (B) Comparison of Treg frequencies in healthy individuals (black), HER2− breast cancer patients (grey) and HER2+ breast cancer patients, either before or during treatment with trastuzumab (white). Shown is the frequency of Treg cells as a percentage of total CD4+ cells. Error bars represent±s.e. P-values < 0.05, as measured by the Mann–Whitney U-test, were considered significant.
To further investigate the role of HER2 status and therapy, we divided patients into HER2-positive or HER2-negative groups. Our data showed that patients with HER2-positive breast cancer had significantly higher frequencies of Treg cells compared with healthy individuals (8.4 vs 6%; P=0.021), whereas the frequency was similar for both HER2-positive and HER2-negative patients (8.4 vs 9.2%; P=0.27; Figure 1B). HER2-positive patients during treatment with trastuzumab had a reduced frequency of Treg cells, compared with that seen at baseline before therapy, but this difference was not statistically significant.
Numbers of Th17 cells are decreased in HER2-positive breast cancer patients
As for the Treg cells, the frequency and absolute number of Th17 cells were measured and compared in healthy individuals and HER2-positive and -negative breast cancer patients (Figure 2 and Supplementary data). Healthy individuals and HER2-negative breast cancer patients had similar Th17 numbers, and in an inverse relationship than that observed in Treg cells, HER2-positive patients had significantly lower frequencies of Th17s compared with healthy and HER2-negative individuals (0.314% compared with 0.748% (P=0.014) and 0.84% (P=0.0088), respectively). We observed that the number of Th17 cells increased upon treatment with trastuzumab (0.314% compared with 0.579%; not significant).
The number of circulatory Th17 cells is decreased in HER2+ breast cancer patients. The frequency of Th17 cells in HER2− breast cancer patients (grey) did not differ from that measured in healthy individuals (black). HER2+ breast cancer patients (white) had significantly lower Th17 frequency compared with both healthy individuals and HER2− patients, and the frequency of Th17s increased during trastuzumab therapy. Th17 frequency was measured as the frequency of CD4+IL17A+ T cells as a percentage of total CD4+ cells. Error bars represent±s.e. P-values < 0.05, as measured by the Mann–Whitney U-test, were considered significant.
The balance of Treg and Th17 cells in PB is altered in HER2-positive breast cancer
To further quantify the relationship between Treg and Th17 numbers in patients with breast cancer, the number of Treg cells to every Th17 cell was calculated as a ratio. Here, we found that HER2-positive breast cancer patients had the highest ratio of Treg : Th17 cells (Figure 3; 35.5 : 1, Treg : Th17 compared with 8 : 1 in healthy donors; P=0.006); HER2-negative patients had a similar balance of Treg : Th17 compared with healthy donors (14 : 1 compared with 8 : 1; P=0.26). Upon treatment with trastuzumab, the Treg : Th17 bias seen in HER2-positive individuals was reduced slightly to 32:1.
The Treg/Th17 bias is highest in HER2+ breast cancer. The Treg : Th17 cell ratio in healthy individuals (black), HER2− breast cancer patients (grey) and HER2+ breast cancer patients treated with trastuzumab (white). The number of Treg cells to Th17s is significantly higher in HER2+ breast cancer patients compared with healthy individuals and HER2− breast cancer patients, with a slight decrease during trastuzumab treatment. The number of Treg cells to every Th17 cell is shown. P-values <0.05, as measured by the Mann–Whitney U-test, were considered significant.
A converse relationship between Treg and Th17 frequency is seen in MBC
We then sought to address whether the increase of Treg cells observed in individuals treated with trastuzumab was a ‘drug-specific’ change dependent on cancer stage. Patients were divided into those who had EBC with no metastatic sites, and patients who had at least one metastatic site (MBC). This revealed individuals with EBC had similar Treg frequencies before, and during trastuzumab therapy (9 and 9.5%, respectively; Figure 4A), with a slight increase following the completion of the course of treatment. The Treg frequency in EBC was comparable to that seen in HER2-negative cancer patients (9 vs 9.2%; P=0.79). Metastatic breast cancer patients had the highest frequency of Treg cells from all populations that we studied (11.1% pre-treatment); this significantly decreased when patients were treated with trastuzumab (7.8%; P=0.039).
The effect of breast cancer stage and trastuzumab on Treg and Th17 frequencies. The frequency of Treg cells and Th17s was measured pre- (white), during (grey) and post (black)-trastuzumab therapy in early breast cancer (EBC) and metastatic breast cancer (MBC) patients. The third histogram shows the relative frequencies seen in healthy and HER2− controls. (A) Treg cells (B) Th17s. Trastuzumab therapy in MBC leads to a statistically significant decrease in the frequency of Treg cells, and conversely, a statistically significant increase in Th17s. Error bars represent±s.e. P-values < 0.05, as measured by the Mann–Whitney U-test, were considered significant.
As for Treg cells, EBC patients had similar Th17 frequencies pre-, during and post-trastuzumab therapy (0.33, 0.45 and 0.32%, respectively). However, in MBC patients, the frequency of Th17s in PB followed an inverse relationship to Treg cell frequency, with numbers significantly increasing during treatment with trastuzumab (0.32 vs 0.64%; P=0.038). The frequency of Th17 cells in HER2-negative breast cancer patients was comparable with that seen in healthy individuals (0.84 vs 0.75%).
When quantifying the relationship between Treg and Th17 frequency, we found that of all populations studied, healthy individuals had the lowest number of Treg cells to every Th17 cell (8.6 : 1). This was significantly lower than that seen in HER2-negative breast cancer (16 : 1; P=0.046), HER2-positive EBC patients (30 : 1; P=0.017) and also HER2-positive MBC patients (40 : 1; P=0.014). In EBC, the Treg : Th17 frequency increased during treatment with trastuzumab, then decreased slightly following completion, but not quite to the levels seen before treatment (pre-treatment: 30 : 1, during: 41.4 : 1, post-treatment: 37.1 : 1). In MBC, however, the Treg : Th17 ratio decreased during treatment (40 : 1 vs 26.8 : 1; P=0.031; Figure 5).
The Treg/Th17 balance. The ratio of Treg : Th17 cells was measured pre- (white), during (grey), and post (black)-trastuzumab therapy in early breast cancer (EBC) and metastatic breast cancer (MBC) patients. The third histogram shows the relative ratios seen in healthy and HER2− controls. The number of Treg cells to every Th17 cell is shown. Error bars represent ±s.e. P-values <0.05, as measured by the Mann–Whitney U-test, were considered significant.
As samples were obtained at multiple time points over the course of trastuzumab treatment, the frequency of cells over time was also measured. The majority of patients remained on the same treatment regime throughout the period of this study, and the frequency of cells (all subsets) remained consistent throughout the specific treatment time course and changed only on starting or stopping the trastuzumab.
Natural killer and NKT cells in breast cancer
In addition to Treg and Th17 cells, the absolute numbers of circulating NK and NKT cells were also measured. This revealed no significant difference between patient populations. The balances and frequencies of NK : NKT cells and Treg : NKT cells were also investigated (as others have performed in inflammatory settings), but again no significant differences were observed.
DISCUSSION
This study is one of the first to document circulating Th17 cells in human cancer patients. Th17 cells have been found in the human gut and PB, and are thought to play a role in inflammatory and autoimmune disorders (Stockinger and Veldhoen, 2007); but their role in cancer, especially in vivo, is however unknown.
We have shown that HER2-positive breast cancer patients have significantly lower frequencies of Th17 cells in their PB compared with both healthy individuals and HER2-negative breast cancer patients. This contradicts a recent study that suggested that although the tumour environment in ovarian cancer was favourable for generation of Th17 cells, the frequencies of Th17 cells in the PB of cancer patients was comparable to that seen in healthy individuals (Miyahara et al, 2008). In addition, we showed that in breast cancer, circulating Treg cells are increased compared with healthy controls (data supported by earlier study (Wolf et al, 2003)). We also observed that although HER2-positive breast cancer patients have a significantly higher frequency of Treg cells compared with healthy donors; the frequency of Treg cells in HER2-positive and -negative individuals did not differ. This finding is in contrast with the data of Perez et al (2007) who found that HER2-negative patients had similar frequencies of Treg cells as healthy donors, but that HER2-positive individuals had significantly higher proportions than both populations.
It is clear that the development of Treg and Th17 cells is closely linked, and both require TGF-β for their differentiation from naïve T cells (Ivanov et al, 2006; Davidson et al, 2007). Recently, the balance of Treg and Th17 cells, and the regulatory balance between these cell types has been of interest to several groups (Elias et al, 2008; Quintana et al, 2008; Veldhoen et al, 2008; Zhou et al, 2008). Although both cell types require TGF-β, other compounds, such as IL-6 and retinoic acid (Nishihara et al, 2007; Wan et al, 2007; Elias et al, 2008), specifically promote either Treg or Th17 cells while suppressing the other cell type.
To further quantify this relationship, we examined the balance between Treg and Th17 cells, by calculating the ratio of Treg : Th17 cells. It was shown that HER2-positive patients had a much higher Treg : Th17 ratio than both HER2-negative individuals and healthy controls. The Treg : Th17 ratio was significantly reduced in MBC patients during trastuzumab therapy, but no such relationship was observed in EBC patients treated with adjuvant trastuzumab. In addition to the Treg : Th17 axis, a regulatory relationship between Treg and NKT cells has also been described (La Cava et al, 2006); the relationship between these cells in the context of breast cancer was therefore examined. We showed no significant differences in NKT cell numbers between all populations, and no significant difference in the Treg : NKT ratio. This suggests that the Treg : Th17 balance ratio is of greater importance than that of the Treg : NKT in breast cancer.
Increasing evidence suggests that the activity of regulatory cells controlling the cellular immune system may have importance in the clinical outcomes of cancer therapies (Bamias et al, 2008). Several studies have shown that higher numbers of Treg cells are associated with progression in a variety of malignancies and can correlate to the poor prognosis (Liyanage et al, 2002; Galon et al, 2006). Here, we suggest that trastuzumab treatment in MBC affects both the numbers of Treg cells and the frequency of circulating Th17 cells.
Most patients in this study were receiving trastuzumab monotherapy; however, a small proportion received trastuzumab in combination with docetaxel or vinorelbine. Although we did not directly address the effect of chemotherapeutic agents alone on Treg and Th17 frequencies, we observed no significant differences between the patient population treated with monotherapy and those treated in combination with chemotherapy. Accordingly, Perez et al (2007) noted no specific difference in Treg frequencies in HER2-negative breast cancer patients receiving the same chemotherapy regimen as patients receiving trastuzumab in combination, and concluded that the differences in Treg frequency were attributed specifically to trastuzumab therapy. Samples were taken from participating patients throughout their treatment, and for most patients, the immune populations measured were similar throughout the period of trastuzumab therapy.
Here, we saw a reduction of Treg numbers, coupled with a converse increase of Th17 cells in the PB of MBC patients receiving trastuzumab therapy. This effect was less pronounced in the adjuvant setting for reasons that remain unclear. Measurement of the frequency of immune cells, such Treg and Th17 cells may prove useful in identifying whether patients are showing any positive response to treatment or not, and this would enable cessation of unnescessary therapy in unresponsive patients. This would be beneficial as trastuzumab is both expensive and can sometimes be associated with serious side effects including cardiotoxicity (Mariani et al, 2008). Whether direct HER2 targeting or an antibody affect is involved in the change in number of Treg and Th17 cells in trastuzumab-treated HER2-positive breast cancer remains unclear. It is possible that trastuzumab may lead to the changes in the cytokine milieu or other factors that may drive expansion of Th17 cells and/or prevent the survival of Treg cells in the body.
This study has been limited by small sample sizes, and functional data have not been collected. Our results, however, support the role of Treg and Th17 cells in trastuzumab therapy. It would appear that further studies are required to confirm the relationship between Treg frequencies and cancer as some of our data conflict with those published previously (Perez et al, 2007). In addition, it may be of interest to further study the effect of trastuzumab therapy in EBC with more patients. It would be of particular interest to observe whether the efficacy of adjuvant therapy in HER2-positive EBC patients could be ultimately predicted by studying the frequency and functionality of patients’ Treg and Th17 cells.
These data are of interest in cancer therapy in general, as harnessing the immune system to improve responses to existing therapies is of increasing importance in clinical trial design of newer immunotherapeutics. The coadministration of trastuzumab along with therapies that either promote Th17 or reduce Treg cells may be a particular direction, with the aim of ultimately improving the prognosis for patients unresponsive to trastuzumab or other therapies.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ahmadzadeh M, Rosenberg SA (2006) IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 107: 2409–2414
Bamias A, Koutsoukou V, Terpos E, Tsiatas ML, Liakos C, Tsitsilonis O, Rodolakis A, Voulgaris Z, Vlahos G, Papageorgiou T, Papatheodoridis G, Archimandritis A, Antsaklis A, Dimopoulos MA (2008) Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFalpha in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol 108: 421–427
Beyer M, Schultze JL (2006) Regulatory T cells in cancer. Blood 108: 804–811
Davidson TS, DiPaolo RJ, Andersson J, Shevach EM (2007) Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol 178: 4022–4026
Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C (2003) Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother 52: 423–428
Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ (2008) Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111: 1013–1020
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960–1964
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133
Javia LR, Rosenberg SA (2003) CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother 26: 85–93
Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W (2007) Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol 178: 6730–6733
La Cava A, Van Kaer L, Fu Dong S (2006) CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol 27: 322–327
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201: 233–240
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169: 2756–2761
Mariani G, Fasolo A, De Benedictis E, Gianni L (2008) Trastuzumab as adjuvant systemic therapy for HER2-positive breast cancer. Nat Clin Pract Oncol 6 (2): 93–104
Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA (2004) Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 103: 1755–1762
McKenzie BS, Kastelein RA, Cua DJ (2006) Understanding the IL-23-IL-17 immune pathway. Trends Immunol 27: 17–23
Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF (2008) Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA 105: 15505–15510
Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P (2002) CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci USA 99: 8832–8837
Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K (2007) Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer 97: 1135–1138
Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, Murakami M, Hirano T (2007) IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol 19: 695–702
Perez SA, Karamouzis MV, Skarlos DV, Ardavanis A, Sotiriadou NN, Iliopoulou EG, Salagianni ML, Orphanos G, Baxevanis CN, Rigatos G, Papamichail M (2007) CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res 13: 2714–2721
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71
Stockinger B, Veldhoen M (2007) Differentiation and function of Th17 T cells. Curr Opin Immunol 19: 281–286
Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A (2004) CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 112: 258–267
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453: 106–109
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189
Wan S, Xia C, Morel L (2007) IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol 178: 271–279
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9: 606–612
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61: 4766–4772
Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR (2008) TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453: 236–240
Acknowledgements
We are grateful to the patients and nurses who assisted with this study, to the ECMC, Roche and the Hammersmith Special Trustees for providing support towards this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Horlock, C., Stott, B., Dyson, P. et al. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br J Cancer 100, 1061–1067 (2009). https://doi.org/10.1038/sj.bjc.6604963
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604963
Keywords
This article is cited by
-
Improved Natural Killer cell activity and retained anti-tumor CD8+ T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy
Journal of Translational Medicine (2015)
-
Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients
Journal of Translational Medicine (2013)
-
Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions
Cancer Immunology, Immunotherapy (2013)
-
Vaccination for the prevention and treatment of breast cancer with special focus on Her-2/neu peptide vaccines
Breast Cancer Research and Treatment (2013)
-
Downregulation of IL-17-producing T cells is associated with regulatory T cell expansion and disease progression in chronic lymphocytic leukemia
Tumor Biology (2013)