Abstract
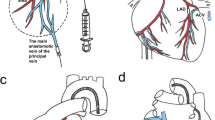
Hydrodynamic gene delivery is an attractive option for non-viral liver gene therapy, but requires evaluation of efficacy, safety and clinically applicable techniques in large animal models. We have evaluated retrograde delivery of DNA to the whole liver via the isolated segment of inferior vena cava (IVC) draining the hepatic veins. Pigs (18–20 kg weight) were given the pGL3 plasmid via two programmable syringe pumps in parallel. Volumes corresponding to 2% of body weight (360–400 ml) were delivered at 100 ml s−1 via a Y connector. The IVC segment pressure, portal venous pressure, arterial pressure, electrocardiogram (ECG) and pulse were monitored. Concurrent studies were performed in rats for interspecies comparisons. The hydrodynamic procedure generated intrahepatic vascular pressures of 101–126 mm Hg, which is ∼4 times higher than in rodents, but levels of gene delivery were ∼200-fold lower. Suprahepatic IVC clamping caused a fall in arterial pressure, with the development of ECG signs of myocardial ischaemia, but these abnormalities resolved rapidly. The IVC segment approach is a clinically acceptable approach to liver gene therapy. However, it is less effective in pigs than in rodents, possibly because of larger liver size or a less compliant connective tissue framework.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Budker V, Zhang G, Knechtle S, Wolff JA . Naked DNA delivered intraportally expresses efficiently in hepatocytes. Gene Therapy 1996; 3: 593–598.
Zhang G, Vargo D, Budker V, Armstrong N, Knechtle S, Wolff JA . Expression of naked plasmid DNA injected into the afferent and efferent vessels of rodent and dog livers. Hum Gene Ther 1997; 8: 1763–1772.
Zhang G, Budker V, Wolff JA . High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 1999; 10: 1735–1737.
Liu F, Song Y, Liu D . Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999; 6: 1258–1266.
Al Dosari MS, Knapp JE, Liu D . Hydrodynamic delivery. Adv Genet 2005; 54: 65–82.
Herweijer H, Wolff JA . Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Therapy 2007; 14: 99–107.
Crespo A, Peydro A, Dasi F, Benet M, Calvete JJ, Revert F et al. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Therapy 2005; 12: 927–935.
Sawyer GJ, Dong X, Whitehorne M, Grehan A, Seddon M, Shah AM et al. Cardiovascular function following acute volume overload for hydrodynamic gene delivery to the liver. Gene Therapy 2007; 14: 1208–1217.
Wisse E, Braet F, Luo D, Vermylen D, Eddouks M, Empsen C et al. Sinusoidal Liver Cells. In: Bircher J, Benhamon JP, McIntyre N, Rizzetto M, Rodes J (eds). Oxford Textbook of Clinical Hepatology, 2nd edn. Oxford University Press, 1999, pp 33–49.
Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ et al. Hydroporation as the mechanism of hydrodynamic delivery. Gene Therapy 2004; 11: 675–682.
Suda T, Gao X, Stolz DB, Liu D . Structural impact of hydrodynamic injection on mouse liver. Gene Therapy 2007; 14: 129–137.
Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003; 80: 148–158.
Zhang X, Dong X, Sawyer GJ, Collins L, Fabre JW . Regional hydrodynamic gene delivery to the rat liver with physiological volumes of DNA solution. J Gene Med 2004; 6: 693–703.
Eastman SJ, Baskin KM, Hodges BL, Chu Q, Gates A, Dreusicke R et al. Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum Gene Ther 2002; 13: 2065–2077.
Herrero MJ, Dasi F, Noguera I, Sanchez M, Moret I, Sanmartin I et al. Mouse and pig nonviral liver gene therapy: success and trials. Gene Ther Mol Biol 2005; 9: 169–180.
Yoshino H, Hashizume K, Kobayashi E . Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Therapy 2006; 13: 1696–1702.
Aliño SF, Herrero MJ, Noguera I, Dasí F, Sánchez M . Pig liver gene therapy by noninvasive interventionist catheterism. Gene Therapy 2007; 14: 334–343.
Inoue S, Hakamata Y, Kaneko M, Kobayashi E . Gene therapy for organ grafts using rapid injection of naked DNA: application to the rat liver. Transplantation 2004; 77: 997–1003.
Ekataksin W, Wake K . Liver units in three dimensions: 1. Organisation of argyrophilic connective tissue skeleton in porcine liver with particular reference to the ‘compound hepatic lobule’. Am J Anat 1991; 191: 113–153.
Carter FJ, Frank TG, Davies PJ, McLean D, Cuschieri A . Measurements and modelling of the compliance of human and porcine organs. Med Image Anal 2001; 5: 231–236.
Higashi N, Ueda H, Yamada O, Oikawa S, Koiwa M, Tangkawattana P et al. Micromorphological characteristics of hepatic sinusoidal endothelial cells and their basal laminae in five different animal species. Okajimas Folia Anat Jpn 2002; 79: 135–142.
Wright PL, Smith KF, Day WA, Fraser R . Small liver fenestrae may explain the susceptibility of rabbits to atherosclerosis. Arteriosclerosis 1983; 3: 344–348.
Walther W, Stein U, Voss C, Schmidt T, Schleef M, Schlag PM . Stability analysis for long-term storage of naked DNA: impact on nonviral in vivo gene transfer. Anal Biochem 2003; 318: 230–235.
Smith AD, Trempe JP . Luminometric quantitation of photinus pyralis firefly luciferase and Escherichia coli beta-galactosidase in blood-contaminated organ lysates. Anal Biochem 2000; 286: 164–172.
Zhang X, Collins L, Sawyer GJ, Dong X, Qiu Y, Fabre JW . In vivo gene delivery via portal vein and bile duct to individual lobes of the rat liver using a polylysine-based nonviral DNA vector in combination with chloroquine. Hum Gene Ther 2001; 12: 2179–2190.
Acknowledgements
This work was funded by the UK Department of Health and the Welton Foundation. We would like to thank Mr Peter Matthews of Medrad for generously providing the syringe pumps, Mr Chris Blacklock of Chalice Medical for advice on pumps, Mr Steve Clarke of Kimal for advice on catheters and high pressure tubing, and Dr Roy Sherwood, Department of Clinical Biochemistry, King's College Hospital, London, for performing the liver function tests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Supplementary information
Rights and permissions
About this article
Cite this article
Fabre, J., Grehan, A., Whitehorne, M. et al. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther 15, 452–462 (2008). https://doi.org/10.1038/sj.gt.3303079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3303079
Keywords
This article is cited by
-
Effects of Fibrotic Tissue on Liver-targeted Hydrodynamic Gene Delivery
Molecular Therapy - Nucleic Acids (2016)
-
Human AAT gene transfer to pig liver improved by using a perfusion isolated organ endovascular procedure
European Radiology (2016)
-
Hemodynamics of a hydrodynamic injection
Molecular Therapy - Methods & Clinical Development (2014)
-
Novel electric power-driven hydrodynamic injection system for gene delivery: safety and efficacy of human factor IX delivery in rats
Gene Therapy (2013)
-
Parameters Affecting Image-guided, Hydrodynamic Gene Delivery to Swine Liver
Molecular Therapy - Nucleic Acids (2013)