Abstract
Background:
Long-chain fatty acids (LCFAs) cross the plasma membrane via a protein-mediated mechanism involving one or more LCFA-binding proteins. Among these, FAT/CD36 has been identified as key LCFA transporter in the heart and skeletal muscle, where it is regulated acutely and chronically by insulin. In skeletal muscle, FAT/CD36 expression and/or subcellular distribution is altered in obesity and type 2 diabetes. There is limited information as to whether the expression of this protein is also altered in subcutaneous and/or visceral adipose tissue depots in human obesity or type 2 diabetes.
Objectives:
To compare (a) the expression of FAT/CD36 in subcutaneous and visceral adipose tissue depots in lean, overweight, and obese individuals and in type 2 diabetics, (b) to determine whether the protein expression of FAT/CD36 in these depots is associated with the severity of insulin resistance (type 2 diabetes>obese>overweight/lean) and (c) whether FAT/CD36 protein expression in these adipose tissue depots is associated with alterations in circulating substrates and hormones.
Subjects:
Subjects who were undergoing abdominal surgery and who were lean (n=10; three men, seven women), overweight (n=10; three men, seven women) or obese (n=7; one man, six women), or who had been diagnosed with type 2 diabetes (n=5; one man, four women) participated in this study.
Measurements:
Subcutaneous and visceral adipose tissue samples, as well as blood samples, were obtained from the subjects while under general anesthesia. Adipose tissue samples were analyzed for FAT/CD36 using Western blotting. Serum samples were analyzed for glucose, insulin, FFA and leptin. BMI was also calculated.
Results:
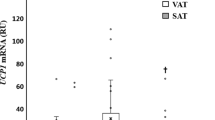
Subcutaneous adipose tissue FAT/CD36 expression was upregulated by +58, +76 and +150% in overweight, obese and type 2 diabetics, respectively. Relative to subcutaneous adipose tissue, visceral adipose tissue FAT/CD36 expression was upregulated in lean (+52%) and overweight subjects (+30%). In contrast, in obese subjects and type 2 diabetics, no difference in FAT/CD36 protein expression was observed between their subcutaneous and visceral adipose tissue depots (P>0.05). The subcutaneous adipose tissue FAT/CD36 expression (R=0.85) and the visceral adipose tissue FAT/CD36 expression (R=0.77) were associated with alteration in BMI and circulating glucose and insulin.
Conclusions:
Subcutaneous adipose tissue FAT/CD36 expression is upregulated in obesity and type 2 diabetes. As FAT/CD36 expression is not different in lean, overweight and obese subjects, and was only increased in type 2 diabetics, it appears that visceral adipose tissue FAT/CD36 may respond in a less dynamic manner to metabolic disturbances than subcutaneous adipose tissue FAT/CD36.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boden G . Free fatty acids, insulin resistance, and type 2 diabetes mellitus. Proc Assoc Am Physicians 1999; 111: 241–248.
Boden G, Chen X . Effects of fat on glucose uptake and utilization patients with non-insulin dependent diabetes mellitus. J Clin Invest 1995; 96: 1261–1268.
Kelley DA, Mandarino LJ . Fuel selection in human skeletal muscle in insulin resistance. A reexamination. Diabetes 2000; 49: 677–683.
Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996; 97: 2859–2865.
Shulman GI . Cellular mechanisms of insulin resistance. J Clin Invest 2000; 106: 171–176.
Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phsophatidylinositol 3-kanise activity in muscle. J Biol Chem 2002; 277: 50230–50236.
Kim Y-B, Shulman GI, Kahn BB . Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase C λ/ζ but not on glycogen synthase kinase-3. J Biol Chem 2002; 277: 32915–32922.
Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JFC et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 2004; 18: 1144–1146.
Luiken JJFP, Arumugam Y, Dyck DJ, Bell RC, Pelsers ML, Turcotte LP et al. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem 2001; 276: 40567–40573.
Luiken JJFP, Arumugam Y, Bell RC, Calles-Escandon J, Tandon NN, Glatz JFC et al. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am J Physiol Endocrinol Metab 2002; 282: 612–621.
Kim JK, Gimeno RE, Higashimori T, Kim H-J, Choi H, Punreddy S et al. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J Clin Invest 2004; 113: 756–763.
Bonen A, Luiken JJFP, Lui S, Dyck DJ, Kiens B, Kristiansen S et al. Palmitate transport and fatty acid transporters in red and white muscles. Am J Physiol Endocrinol Metab 1998; 275: E471–E478.
Steinberg GR, Dyck DJ, Calles-Escandon J, Tandon NN, Luiken JJFP, Glatz JF et al. Chronic leptin administration decreases fatty acid uptake and fatty acid transporters in rat skeletal muscle. J Biol Chem 2002; 277: 8854–8860.
Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Frieda S et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999; 274: 19055–19062.
Coburn CT, Knapp Jr FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA . Defective uptake and utilization of long chain fatty acids in muscle and adipose tissue of CD36 knockout mice. J Biol Chem 2000; 275: 32523–32529.
Bonen A, Campbell SE, Benton CR, Chabowski A, Coort SL, Han XX et al. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc 2004; 63: 245–249.
Goudriaan JR, Dahlmans VE, Teusink B, Ouwens DM, Febbraio M, Maassen JA et al. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res 2003; 44: 2270–2277.
Koonen DPY, Benton CR, Arumugam Y, Tandon NN, Calles-Escandon J, Glatz JFC et al. Different mechanism can alter fatty acid transport when muscle contractile activity is chronically altered. Am J Physiol Endocrinol Metab 2004; 286: 1042–1049.
Gertow K, Bellanda M, Eriksson P, Boquist S, Hamsten A, Sunnerhagen M et al. Genetic and structural evaluation of fatty acid transport protein-4 in relation to markers of the insulin resistance syndrome. J Clin Endocrinol Metab 2004; 89: 392–399.
Fisher RM, Hoffstedt J, Hotamisligil GS, Thorne A, Ryden M . Effects of obesity and weight loss on the expression of proteins involved in fatty acid metabolism in human adipose tissue. Int J Obes Relat Metab Disord 2002; 26: 1379–1385.
Asakawa H, Tokunaga K, Kawakami F . Relationship of abdominal fat with metabolic disorders in diabetes mellitus patients. Diabetes Res Clin Pract 2002; 55: 139–149.
Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH . Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000; 278: E941–E948.
Matsuno K, Diaz-Ricard M, Montgomery RR, Aster T, Jamieson GA, Tandon NN . Inhibition of platelet adhesion to collagen by monoclonal anti CD36 antibodies. Br J Haematol 1996; 92: 960–967.
Bergmeyer HU . Methods of Enzymatic Analysis. Academic Press: New York, 1983.
Gertow K, Pietilainen KH, Yki-Jarvinen H, Kaprio J, Rissanen A, Eriksson P et al. Expression of fatty-acid-handling proteins in human adipose tissue in relation to obesity and insulin resistance. Diabetologia 2004; 47: 1118–1125.
Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ et al. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am J Physiol Endocrinol Metab 2004; 287: E781–E789.
Rome S, Clement K, Rabasa-Lhoret R, Loizon E, Poitou C, Barsh GS et al. Microarray profiling of human skeletal muscle reveals that insulin regulates ∼800 genes during a hyperinsulinemic clamp. J Biol Chem 2003; 278: 18063–18068.
Coort SL, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF, Luiken JJ . Divergent effects of rosiglitazone on protein-mediated fatty acid uptake in adipose and in muscle tissues of Zucker rats. J Lipid Res 2005; 46: 1295–1302.
Chambrier C, Aouifi A, Bon C, Saudin F, Paturel B, Bouletreau P . Effects of intraoperative glucose administration on circulating metabolites and nitrogen balance during prolonged surgery. J Clin Anesthiol 1999; 11: 646–651.
Geisser W, Schreiber M, Hofbauer H, Lattermann R, Fussel S, Wachter U et al. Sevoflurane versus isoflurane – anaesthesia for lower abdominal surgery. Effects on perioperative glucose metabolism. Acta Anaesthesiol Scand 2003; 47: 174–179.
Lattermann R, Schricker T, Georgieff M, Schreiber M . Low dose clonidine premedication accentuates the hyperglycemic response to surgery. Can J Anaesthiol 2001; 48: 755–759.
Acknowledgements
These studies were supported by grants form the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Netherlands Heart Foundation (200.156) and the Canada Research Chair program. JFC Glatz is Netherlands Heart Foundation Professor of Cardiac Metabolism. JJFP Luiken is a recipient of a VIDI-Innovation Research Grant from the Netherlands Organization for Scientific Research (NOW-ZonMw Grant 016.036.305). A Bonen is the Canada Research Chair in Metabolism and Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonen, A., Tandon, N., Glatz, J. et al. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes 30, 877–883 (2006). https://doi.org/10.1038/sj.ijo.0803212
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803212
Keywords
This article is cited by
-
Visceral mesenchymal stem cells from type 2 diabetes donors activate triglycerides synthesis in healthy adipocytes via metabolites exchange and cytokines secretion
International Journal of Obesity (2023)
-
Stomatin modulates adipogenesis through the ERK pathway and regulates fatty acid uptake and lipid droplet growth
Nature Communications (2022)
-
Maternal obesity during pregnancy leads to adipose tissue ER stress in mice via miR-126-mediated reduction in Lunapark
Diabetologia (2021)
-
TLR4 inhibitor TAK-242 attenuates the adverse neural effects of diet-induced obesity
Journal of Neuroinflammation (2018)
-
Agaricus bisporus supplementation reduces high-fat diet-induced body weight gain and fatty liver development
Journal of Physiology and Biochemistry (2018)