Abstract
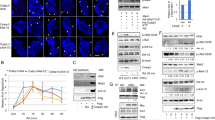
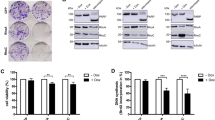
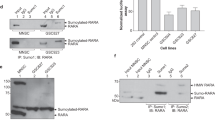
Retinoic acid receptor-β (RARβ) and signal transducer and activator of transcription 1 (STAT1) are important mediators of the antiproliferative and apoptotic actions of retinoids and cytokines/growth factors, respectively. Expression of both RARβ and STAT1 is lost in most breast cancer cell lines but it can be induced by retinoids in estrogen receptor-positive cells. We investigated a possible functional connection between these two mediators and present evidence supporting RARβ as a tumor suppressor. First, by using different receptor-selective retinoids, we demonstrated that RARβ induction in MCF-7 cells by all-trans-retinoic acid (atRA) was associated with the activation of STAT1 gene transcription. The direct involvement of RARβ in atRA-induced STAT1 gene activation was further demonstrated by showing that transfection with an anti-sense RARβ construct blocked atRA-induced STAT1 expression in MCF-7 cells whereas introduction of a sense-RARβ construct resulted in STAT1 induction by atRA in MDA-MB 231 cells. In addition, we showed that STAT1 was phosphorylated/activated under atRA treatment of MCF-7 cells; this process required the involvement of RARβ and protein synthesis. STAT1 phosphorylation/activation was accompanied by increased tyrosine kinase activity that was not due to the activation of JAK1, JAK2 or Tyk 2, suggesting the possible involvement of an unidentified tyrosine kinase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Berard J, Laboune F, Mukuna M, Masse S, Kothary R and Bradley WE. . 1996 FASEB J. 10: 1091–1097.
Bromberg JF, Horvath CM, Wen Z, Schreiber RD and Darnell Jr JE. . 1996 Proc. Natl. Acad. Sci. USA 93: 7673–7678.
Chin YE, Kitagawa M, Su WCS, You ZH, Iwamoto Y and Fu XY. . 1996 Science 272: 719–722.
Darnell Jr JE, Kerr IM and Stark JM. . 1994 Science 264: 1415–1421.
Fitzgerald P, Teng M, Chandraratna RAS, Heyman RA and Allegretto EA. . 1997 Cancer Res. 57: 2642–2650.
Geier A, Haimshon M, Beery R, Hemi R and Lunenfeld B. . 1992 In Vitro Cell. Dev. Biol. 28: 725–729.
Gerbert JF, Moghal N, Frangioni JV, Sugarbaker DJ and Neel BJ. . 1991 Oncogene 6: 1859–1868.
Gianni M, Li MC, Terao M, Rambaldi A and Garattini E. . 1995 Biochem. Biophys. Res. Commun. 208: 846–854.
Gianni M, Terao M, Fortino I, Licalzi M, Viggiano V, Barbui T, Rambaidi A and Garattini E. . 1997 Blood 89: 1001–1012.
Higuchi T, Hannigan GE, Malkan D, Yeger H and Williams BR. . 1991 Cancer Res. 51: 3958–3964.
Houle B, Leduc F and Bradley WEC. . 1991 Genes Chromosomes Cancer 3: 358–366.
Kalvakolanu DV and Borden EC. . 1996 Cancer Invest. 14: 25–53.
Kolla V, Lindner DJ, Weihua X, Borden EC and Kalvakolanu DV. . 1996 J. Biol. Chem. 271: 10508–10514.
Kumar A, Commane M, Flickinger TW, Horvath CM and Stark GR. . 1997 Science 278: 1630–1632.
Langenfeld J, Kiyokawa DS, Boyle J and Dmitrovsky E. . 1997 Proc. Natl. Acad. Sci. USA 94: 12071–12074.
Liu Y, Lee MO, Wang HG, Li Y, Hashimoto Y, Klaus M, Reed JC and Zhang XK. . 1997 Mol. Cell. Biol. 16: 1138–1149.
Mangelsdorf DJ, Umesono K and Evans RM. . 1994 In Sporn MB, Roberts AB and Goodman DS, (eds). The retinoids, 2nd ed. 319–349, Raven Press: New York.
Matikainen S, Ronni T, Lehtonen A, Sareneva T, Melen K, Nordling S, Levy DE and Julkunen I. . 1997 Cell Growth Differen. 8: 687–698.
Schindler C and Darnell Jr JE. . 1995 Annu. Rev. Biochem. 64: 621–651.
Seewaldt VL, Johnson BS, Parker MB, Collins SJ and Swisshelm K. . 1995 Cell Growth Differen. 6: 1077–1088.
Shang Y, Baumrucker CR and Green MH. . 1998 J. Biol. Chem. 273: 30608–30613.
Sheikh MS, Shao AM, Li XS, Dawson M, Jetten AM and Fontana JA. . 1994 J. Biol. Chem. 269: 21440–21447.
Si SP, Lee X, Tsou HC, Buchsbaum R, Tibaduiza E and Peacocke M. . 1996 Exp. Cell Res. 223: 102–111.
Sun WH, Pabon CC, Alsayed Y, Huang PP, Jandeska S, Uddin S, Platanias LC and Rosen ST. . 1998 Blood 91: 570–576.
Swisshelm K, Ryan K, Lee X, Tsou HC, Peacocke M and Sager R. . 1994 Cell Growth Differen. 5: 133–141.
Widschwendter M, Berger J, Daxenbichler G, Muller-Holzner E, Widschwendter A, Mayr A, Marth C and Zeiimet AG. . 1997 Cancer Res. 57: 4158–4161.
Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ and Ralph SJ. . 1997 J. Biol. Chem. 272: 28779–28785.
Zhang XK, Liu Y and Lee MO. . 1996 Mutation Res. 350: 267–277.
Acknowledgements
The authors are indebted to Drs R M Evans (Gene Expression Laboratory, Salk Institute for Biological Studies, La Jolla, CA, USA), X-K Zhang (La Jolla Cancer Research Center, La Jolla, CA, USA) and J E Darnell, Jr (Laboratory of Molecular Cell Biology, The Rockefeller University, New York, NY, USA) for providing plasmid constructs used in this experiment. We also thank Anne Gibson for technical assistance and Joanne Balmer Green for helping with manuscript preparation. This work was supported by grants from the National Institutes of Health (R01-HD32500 to MH Green) and the Pennsylvania State University Agricultural Experiment Station (to CR Baumrucker).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shang, Y., Baumrucker, C. & Green, M. The induction and activation of STAT1 by all-trans-retinoic acid are mediated by RARβ signaling pathways in breast cancer cells. Oncogene 18, 6725–6732 (1999). https://doi.org/10.1038/sj.onc.1203084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203084
Keywords
This article is cited by
-
Direct and indirect effects of IFN-α2b in malignancy treatment: not only an archer but also an arrow
Biomarker Research (2022)
-
Glycogen metabolism regulates macrophage-mediated acute inflammatory responses
Nature Communications (2020)
-
Analysis of the interplay between all-trans retinoic acid and histone deacetylase inhibitors in leukemic cells
Archives of Toxicology (2017)
-
The HER2 inhibitor TAK165 Sensitizes Human Acute Myeloid Leukemia Cells to Retinoic Acid-Induced Myeloid Differentiation by activating MEK/ERK mediated RARα/STAT1 axis
Scientific Reports (2016)
-
Synergy between RA and TLR3 promotes type I IFN-dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells
Cell Death & Disease (2013)