Abstract
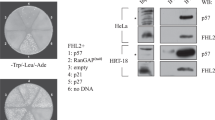
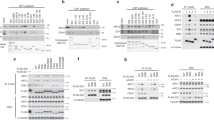
Akt, a proto-oncogene that encodes a cytosolic serine/threonine kinase, can phosphorylate and modulate the activity of several proteins involved in cellular metabolism and survival. Recently, two mammalian highly related forkhead transcription factors FKHRL1 and AFX and their nematode homologue Daf-16 have been found to be targets of this kinase. Here we show that Akt, but not inactive Akt, represses the transcriptional activity of FKHR, another member of the forkhead family. FKHR mutants with alanine substitutions at three Akt phosphorylation consensus sites (T24, S256 and S319) were inhibited by Akt, but mutation of all three sites rendered FKHR resistant to suppression. By contrast, the transcriptional activity of the oncogenic PAX3 – FKHR fusion protein, containing two consensus phosphorylation sites, was not inhibited by Akt. Importantly, Akt inhibited the translocation of FKHR to the nucleus, providing a mechanism by which Akt might regulate the transcriptional activity of FKHR. Consistent with this model, the localization of the PAX3 – FKHR fusion protein was nuclear and was not altered by Akt. These results provide evidence that Akt inhibits the transcriptional activity of FKHR by controlling its trafficking into the nucleus and that oncogenic PAX3 – FKHR can escape this negative regulation by Akt.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alessi DR, Caudwell FB, Andjelkovich M, Hemmings BA and Cohen P. . 1996 FEBS Lett. 399: 333–338.
Barr FG, Galili N, Holick J, Biegel JA, Rovera G and Emanuel BS. . 1993 Nat. Genet. 3: 113–117.
Bellacosa A, Testa JR, Stall SP and Tsichis PN. . 1991 Science 254: 274–277.
Bennicelli JL, Edwards RH and Barr FG. . 1996 Proc. Natl. Acad. Sci. USA 93: 5455–5459.
Bennicelli JL, Fredericks WJ, Wilson RB, Rauscher Jr F and Barr FG. . 1995 Oncogene 11: 119–130.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME. . 1999 Cell 96: 857–868.
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S and Reed JC. . 1998 Science 282: 1318–1321.
Cichy SB, Uddin S, Danilkovich A, Guo S, Klippel A and Unterman TG. . 1998 J. Biol. Chem. 273: 6482–6487.
Coffer PJ, Jin J and Woodgett JR. . 1998 Biochem. J. 335: 1–13.
Cross DAE, Alessi DR, Cohen P, Andjelkovich M and Hemmings BA. . 1995 Nature 378: 785–789.
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y and Greenberg ME. . 1997 Cell 91: 231–241.
del Peso L, Gonzalez-Garcia M, Page C, Herrera R and Núñez G. . 1997 Science 278: 687–689.
Franke TF, Yang S-I, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR and Tsichlis PN. . 1995 Cell 81: 727–736.
Fredericks WJ, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr FG and Rauscher Jr F. . 1995 Mol. Cell. Biol. 15: 1522–1535.
Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJD, Emanuel BS, Rovera G and Barr FG. . 1993 Nat. Gen. 5: 230–235.
Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL and Burgering BM. . 1991 Nature 398: 630–634.
Lam PY, Sublett GE, Hollenbach AD and Roussel MF. . 1999 Mol. Cell. Biol. 19: 594–601.
Lin K, Dorman JB, Rodan A and Kenyon C. . 1997 Science 278: 1319–1322.
Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA and Ruvkun G. . 1997 Nature 389: 994–999.
Paradis S and Ruvkun G. . 1998 Genes Dev. 12: 2488–2498.
Pierrou S, Hellqvist M, Samuelsson L, Enerback S and Carlsson P. . 1994 EMBO J. 13: 5002–5012.
Shapiro DN, Sublett JE, Li B, Downing JR and Naeve CW. . 1993 Cancer Res. 53: 5108–5112.
Sublett JE, Jeon IS and Shapiro DN. . 1995 Oncogene 11: 545–552.
Wang W, Kumar P, Wang W, Epstein J, Helman L, Moore JV and Kumar S. . 1998 Cancer Res. 58: 4426–4433.
Acknowledgements
We thank Y Hu, N Inohara and MA Benedict for critical review of the manuscript and experimental suggestions. We thank BA Hemmings and TF Franke for Akt plasmids. G Núñez is a recipient of a Research Career Development Award CA-64421 from the National Institutes of Health. The research was supported by a grant from the JP McCarthy Foundation to G Núñez. L dePeso was supported by a training grant from the University of Michigan/NCI Program. VM González was supported by a Fellowship from the Spanish Ministry of Education and Science. R Hernández was supported by the NIH grant #P01-CA75136-01A1. FG Barr was supported by the NIH grant #R01-CA64202.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
del Peso, L., González, V., Hernández, R. et al. Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene 18, 7328–7333 (1999). https://doi.org/10.1038/sj.onc.1203159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203159
Keywords
This article is cited by
-
Regulation of brain endothelial cell physiology by the TAM receptor tyrosine kinase Mer
Communications Biology (2023)
-
Rhabdomyosarcoma
Nature Reviews Disease Primers (2019)
-
The roles of FoxOs in modulation of aging by calorie restriction
Biogerontology (2015)
-
Modulation of oxidative stress as an anticancer strategy
Nature Reviews Drug Discovery (2013)
-
Alveolar rhabdomyosarcoma-associated proteins PAX3/FOXO1A and PAX7/FOXO1A suppress the transcriptional activity of MyoD-target genes in muscle stem cells
Oncogene (2013)