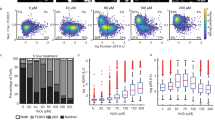
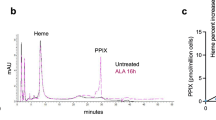
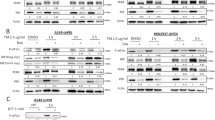
Abstract
A recently identified class of signaling factors uses critical cysteine motif(s) that act as redox-sensitive ‘sulfhydryl switches’ to reversibly modulate specific signal transduction cascades regulating downstream proteins with similar redox-sensitive sites. For example, signaling factors such as redox factor-1 (Ref-1) and transcription factors such as the AP-1 complex both contain redox-sensitive cysteine motifs that regulate activity in response to oxidative stress. The mammalian thioredoxin reductase-1 (TR) is an oxidoreductase selenocysteine-containing flavoprotein that also appears to regulate multiple downstream intracellular redox-sensitive proteins. Since ionizing radiation (IR) induces oxidative stress as well as increases AP-1 DNA-binding activity via the activation of Ref-1, the potential roles of TR and thioredoxin (TRX) in the regulation of AP-1 activity in response to IR were investigated. Permanently transfected cell lines that overexpress wild type TR demonstrated constitutive increases in AP-1 DNA-binding activity as well as AP-1-dependent reporter gene expression, relative to vector control cells. In contrast, permanently transfected cell lines expressing a TR gene with the active site cysteine motif deleted were unable to induce AP-1 activity or reporter gene expression in response to IR. Transient genetic overexpression of either the TR wild type or dominant-negative genes demonstrated similar results using a transient assay system. One mechanism through which TR regulates AP-1 activity appears to involve TRX sub-cellular localization, with no change in the total TRX content of the cell. These results identify a novel function of the TR enzyme as a signaling factor in the regulation of AP-1 activity via a cysteine motif located in the protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Abate C, Patel L, Rauscher III FJ, Curran T . 1990 Science 249: 1157–1161
Abate C, Marshak DR, Curran T . 1991 Oncogene 6: 2179–2185
Becker K, Gromer S, Schirmer RH, Müller S . 2000 Eur. J. Biochem. 267: 6118–6125
Beetz A, Peter RU, Oppel T, Kaffenberger W, Rupec RA, Meyer M, van Beuningen D, Kind P, Messer G . 2000 Int. J. Radiat. Biol. 76: 1443–1453
Berggren M, Gallegos A, Gasdaska JR, Gasdaska PY, Warneke J, Powis G . 1996 Anticancer Res. 16: 3459–3466
Curry HA, Clemens RA, Shah S, Bradbury CM, Botero A, Goswami P, Gius D . 1999 J. Biol. Chem. 274: 23061–23067
Dignam JD . 1990 Meth. Enzymol. 182: 194–203
Gallegos A, Gasdaska JR, Taylor CW, Paine-Murrieta GD, Goodman D, Gasdaka PY, Berggren M, Briehl MM, Powis G . 1996 Cancer Res. 56: 5765–5770
Franza Jr BR, Rauscher III FJ, Josephs SF, Curran T . 1988 Science 239: 1150–1153
Gius D, Cao XM, Rauscher III FJ, Cohen DR, Curran T, Sukhatme VP . 1990 Mol. Cell. Biol. 10: 4243–4255
Hallahan DE, Gius D, Kuchibhotla J, Sukhatme V, Kufe DW, Weichselbaum RR . 1993 J. Biol. Chem. 268: 4903–4907
Halliwell B, Gutteridge JM . 1988 Hum. Toxicol. 7: 7–13
Halliwell B, Gutteridge JM . 1990 Methods Enzymol. 186: 1–85
Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J . 1999 J. Biol. Chem 274: 27891–27897
Hirota K, Matsui M, Murata M, Takashima Y, Cheng FS, Itoh T, Fukuda K, Yodoi J . 2000a Biochem. Biophys. Res. Comm. 274: 177–182
Hirota K, Nakamura H, Arai T, Ishii H, Bai J, Itoh T, Fukuda K, Yodoi J . 2000b Biochem. Biophys. Res. Comm. 275: 825–830
Holbrook NJ, Fornace Jr AJ . 1991 New Biol. 3: 825–833
Holmgren A, Bjornstedt M . 1995 Meth. Enzymol. 252: 199–208
Hu J, Ma X, Lindner DJ, Karra S, Hofmann ER, Reddy SP, Kalvakolanu DV . 2001 Oncogene 20: 4235–4248
Karin M, Smeal T . 1992 Trends Biochem. Sci 17: 418–422
Kerppola T, Curran T . 1995 Nature 373: 199–200
Kerr LD, Inoue J, Verma IM . 1992 Curr. Opin. Cell Biol. 4: 496–501
Kirkpatrick DL, Ehrmantraut G, Stettner S, Kunkel M, Powis G . 1997 Oncol. Res. 9: 351–356
Maity A, McKenna WG, Muschel RJ . 1994 Radiother. Oncol. 31: 1–13
Ma X, Karra S, Lindner DJ, Hu J, Reddy SP, Kimchi A, Yodoi J, Kalvakolanu DV . 2001 Oncogene 20: 3703–3715
Martin M, Vozenin MC, Gault N, Crechet F, Pfarr CM, Lefaix JL . 1997 Oncogene 18: 981–989
Mustacich D, Powis G . 2000 Biochem. J. 346: 1–8
Oberley TD, Verwiebe E, Zhong W, Kang SW, Rhee SG . 2001 Free Radic. Biol. Med. 30: 412–424
Poole LB, Reynolds CM, Wood ZA, Karplus PA, Ellis HR, Calzi ML . 2000 Eur. J. Biochem. 267: 6126–6133
Powis G, Kirkpatrick DL, Angulo M, Baker A . 1998 Chem. Biol. Interact. 112: 23–34
Sasada T, Sono H, Yodoi J . 1996 J. Toxicol. Sci. 21: 285–287
Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ . 2000 Ann. NY Acad. Sci. 899: 349–362
Storz G, Tartaglia LA, Ames BN . 1990 Science 248: 189–194
Tuttle SW, Varnes ME, Mitchell JB, Biaglow JE . 1992 Int. J. Radiat. Onc. Biol. Phys. 22: 671–675
Wei SJ, Botero A, Hirota K, Bradbury CM, Markovina S, Laszlo A, Spitz DR, Goswami PC, Yodoi J, Gius D . 2000 Cancer Res. 60: 6688–6695
Williams CH, Arscott DL, Müller S, Lennon BW, Ludwig ML, Wang PM, Veine DM, Becker K, Heiner Schirmer R . 2000 Eur. J. Biochem. 267: 6110–6119
Xanthoudakis S, Curran T . 1992 EMBO J. 11: 653–664
Xanthoudakis S, Miao G, Wang F, Pan Y-C, Curran T . 1992 EMBO J. 11: 3323–3335
Xanthoudakis S, Curran T . 1996 Adv. Exp Med. Biol. 387: 69–75
Acknowledgements
The authors are indebted to Dr Jeffery Russell for the invaluable technical expertise offered at stages throughout this project. D Gius was supported by grants from the National Institute of Health (1 K08 CA72602-01), and the American Cancer Society (ACS-IRG-58-010-43 and ACS RPG-00-292-01-TBE). DR Spitz was supported by NIH R01 HL51469. NIH CA69593 supported P Goswami. DV Kalvakolanu was supported by grants from the National Institute of Health (R01 CA 78282 and CA 71401).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karimpour, S., Lou, J., Lin, L. et al. Thioredoxin reductase regulates AP-1 activity as well as thioredoxin nuclear localization via active cysteines in response to ionizing radiation. Oncogene 21, 6317–6327 (2002). https://doi.org/10.1038/sj.onc.1205749
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205749
Keywords
This article is cited by
-
Dynamic proteomics reveals bimodal protein dynamics of cancer cells in response to HSP90 inhibitor
BMC Systems Biology (2017)
-
MiR-23-TrxR1 as a novel molecular axis in skeletal muscle differentiation
Scientific Reports (2017)
-
Identification of potential molecular biomarkers in response to thioredoxin reductase 1 deficiency under nickel exposure
BioChip Journal (2012)
-
Synergistic genotoxic effect between gene and environmental pollutant: Oxidative DNA damage induced by thioredoxin reductase 1 silencing under nickel treatment
Molecular & Cellular Toxicology (2011)
-
c-Jun-NH2 terminal kinase (JNK)-mediates AP-1 activation by thioredoxin: phosphorylation of cJun, JunB, and Fra-1
Molecular and Cellular Biochemistry (2010)