Abstract
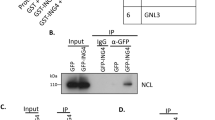
EBP1 was identified as a protein that interacts with the ErbB-3 receptor and possibly contributes to transducing growth regulatory signals. The existence of EBP1 homologs across species from simple eukaryotes to humans and its wide tissue expression pattern suggest that EBP1 acts as a general signaling molecule. We provide evidence that EBP1 is localized to the cytoplasm and to the nucleolus, and that its nucleolar localization requires amino-acid sequences present at both the amino- and carboxy-terminus of the molecule. We also show that EBP1 overexpression inhibits proliferation of human fibroblasts, and that this effect is linked to its nucleolar localization. Using mass spectrometry we demonstrate that EBP1 is part of ribonucleoprotein complexes and associates with different rRNA species. It is becoming clear that cell growth and proliferation are actively coordinated with rRNA processing and ribosome assembly. Our findings indicate that EBP1 is a nucleolar growth-regulating protein, and we propose that it could represent a new link between ribosome biosynthesis and cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Andersen JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M and Lamond AI . (2002). Curr. Biol., 12, 1–11.
Borer RA, Lehner CF, Eppenberger HM and Nigg EA . (1989). Cell, 56, 379–390.
Bourbon HM, Bugler B, Caizergues-Ferrer M, Amalric F and Zalta JP . (1983). Mol. Biol. Rep., 9, 39–47.
Bouvet P, Diaz JJ, Kindbeiter K, Madjar JJ and Amalric F . (1998). J. Biol. Chem., 273, 19025–19029.
Catez F, Erard M, Schaerer-Uthurralt N, Kindbeiter K, Madjar JJ and Diaz JJ . (2002). Mol. Cell. Biol., 22, 1126–1139.
Dragon F, Gallagher JE, Compagnone-Post PA, Mitchell BM, Porwancher KA, Wehner KA, Wormsley S, Settlage RE, Shabanowitz J, Osheim Y, Beyer AL, Hunt DF and Baserga SJ . (2002). Nature, 417, 967–970.
Eisenhaber F, Wechselberger C and Kreil G . (2001). Trends Biochem. Sci., 26, 345–347.
Fatica A and Tollervey D . (2002). Curr. Opin. Cell Biol., 14, 313–318.
Filipowicz W and Pogacic V . (2002). Curr. Opin. Cell Biol., 14, 319–327.
Granneman S, Gallagher JE, Vogelzangs J, Horstman W, van Venrooij WJ, Baserga SJ and Pruijn GJ . (2003). Nucleic Acids Res., 31, 1877–1887.
Guarente L . (1997). Genes Dev., 11, 2449–2455.
Hayano T, Yanagida M, Yamauchi Y, Shinkawa T, Isobe T and Takahashi N . (2003). J. Biol. Chem., 278, 34309–34319.
Hellen CU and Sarnow P . (2001). Genes Dev., 15, 1593–1612.
Hernandez-Verdun D, Roussel P and Gebrane-Younes J . (2002). J. Cell Sci., 115, 2265–2270.
Kiss T . (2002). Cell, 109, 145–148.
Kressler D, Linder P and de La Cruz J . (1999). Mol. Cell. Biol., 19, 7897–7912.
Lamartine J, Seri M, Cinti R, Heitzmann F, Creaven M, Radomski N, Jost E, Lenoir GM, Romeo G and Sylla BS . (1997). Cytogenet. Cell Genet., 78, 31–35.
Lessor TJ, Yoo JY, Xia X, Woodford N and Hamburger AW . (2000). J. Cell. Physiol., 183, 321–329.
Lixin R, Efthymiadis A, Henderson B and Jans DA . (2001). Biochem. Biophys. Res. Commun., 284, 185–193.
Llanos S, Clark PA, Rowe J and Peters G . (2001). Nat. Cell Biol., 3, 445–452.
Mann M and Talbo G . (1996). Curr. Opin. Biotechnol., 7, 11–19.
Mitchell SA, Spriggs KA, Coldwell MJ, Jackson RJ and Willis AE . (2003). Mol. Cell, 11, 757–771.
Mosmann T . (1983). J. Immunol. Methods, 65, 55–63.
Nissan TA, Bassler J, Petfalski E, Tollervey D and Hurt E . (2002). EMBO J., 21, 5539–5547.
Pederson T . (1998). J. Cell Biol., 143, 279–281.
Pestov DG, Strezoska Z and Lau LF . (2001). Mol. Cell. Biol., 21, 4246–4255.
Pilipenko EV, Pestova TV, Kolupaeva VG, Khitrina EV, Poperechnaya AN, Agol VI and Hellen CU . (2000). Genes Dev., 14, 2028–2045.
Pinol-Roma S . (1999). Mol. Biol. Cell, 10, 77–90.
Rizos H, Darmanian AP, Mann GJ and Kefford RF . (2000). Oncogene, 19, 2978–2985.
Schafer T, Strauss D, Petfalski E, Tollervey D and Hurt E . (2003). EMBO J., 22, 1370–1380.
Scheer U and Hock R . (1999). Curr. Opin. Cell Biol., 11, 385–390.
Scherl A, Coute Y, Deon C, Calle A, Kindbeiter K, Sanchez JC, Greco A, Hochstrasser D and Diaz JJ . (2002). Mol. Biol. Cell, 13, 4100–4109.
Schneiter R, Kadowaki T and Tartakoff AM . (1995). Mol. Biol. Cell, 6, 357–370.
Shaw PJ and Jordan EG . (1995). Annu. Rev. Cell Dev. Biol., 11, 93–121.
Srivastava M and Pollard HB . (1999). FASEB J., 13, 1911–1922.
Strezoska Z, Pestov DG and Lau LF . (2002). J. Biol. Chem., 277, 29617–29625.
Sugimoto M, Kuo ML, Roussel MF and Sherr CJ . (2003). Mol. Cell, 11, 415–424.
Warner JR . (1999). Trends Biochem. Sci., 24, 437–440.
Warner JR . (2001). Cell, 107, 133–136.
Wehner KA and Baserga SJ . (2002). Mol. Cell, 9, 329–339.
Xia X, Cheng A, Lessor T, Zhang Y and Hamburger AW . (2001a). J. Cell. Physiol., 187, 209–217.
Xia X, Lessor TJ, Zhang Y, Woodford N and Hamburger AW . (2001b). Biochem. Biophys. Res. Commun., 289, 240–244.
Yanagida M, Shimamoto A, Nishikawa K, Furuichi Y, Isobe T and Takahashi N . (2001). Proteomics, 1, 1390–1404.
Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA and Hamburger AW . (2000). Br. J. Cancer, 82, 683–690.
Zhang Y, Fondell JD, Wang Q, Xia X, Cheng A, Lu ML and Hamburger AW . (2002). Oncogene, 21, 5609–5618.
Zhang Y, Woodford N, Xia X and Hamburger AW . (2003). Nucleic Acids Res., 31, 2168–2177.
Acknowledgements
We thank Emanuela Colombo for the anti-NPM antibodies. We acknowledge Pietro Transidico for confocal microscope analysis, and Ivan Muradore for FACS analysis and IEO-IFOM sequencing unit. This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Consiglio nazionale delle Ricerche (CNR), Fondazione Italiana per la Ricerca sul Cancro (FIRC) and Telethon to GFD. MS and MD were supported by fellowships from FIRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information accompanies the paper on Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Squatrito, M., Mancino, M., Donzelli, M. et al. EBP1 is a nucleolar growth-regulating protein that is part of pre-ribosomal ribonucleoprotein complexes. Oncogene 23, 4454–4465 (2004). https://doi.org/10.1038/sj.onc.1207579
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207579
Keywords
This article is cited by
-
The roles of multifunctional protein ErbB3 binding protein 1 (EBP1) isoforms from development to disease
Experimental & Molecular Medicine (2020)
-
Investigating the role of Ebp1 in Chandipura virus infection
Journal of Biosciences (2019)
-
Proteomics Profiling of Host Cell Response via Protein Expression and Phosphorylation upon Dengue Virus Infection
Virologica Sinica (2019)
-
Targeting the pancreatic β-cell to treat diabetes
Nature Reviews Drug Discovery (2014)
-
Upregulated Expression of Ebp1 Contributes to Schwann Cell Differentiation and Migration After Sciatic Nerve Crush
Journal of Molecular Neuroscience (2014)