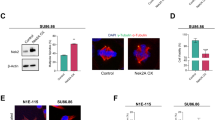
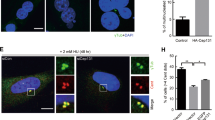
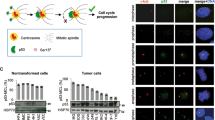
Abstract
The centrosome modulates spindle formation and plays a critical role in guiding proper segregation of chromosomes during cell division. Centrosome aberrations, frequently seen in human tumors, may cause abnormal chromosome segregation and contribute to malignant transformation. To explore the components of the centrosomes, we previously identified a novel centrosomal protein called Su48. To further characterize the Su48-containing protein ensemble in the centrosome, we performed yeast two-hybrid screens and isolated a number of Su48-interacting molecules, including the centrosomal protein Nde1. Here, we demonstrate that Su48 can associate with Nde1. Moreover, we found that Nde1 is subjected to phosphorylation in vivo. In particular, we identified six putative Cdc2 phosphorylation sites in Nde1 and found that alteration of these sites diminishes phosphorylation by Cdc2 in vitro and affects the stability of Su48-Nde1 interactions and the centrosomal localization of Nde1. Ablation of Nde1 by gene specific small interfering RNA causes mitotic delay and cell death, coupled with a modest decrease in the incidence of the cells that harbor excessive centrosomes. Collectively, our findings indicate that Nde1 can form a protein complex with Su48 in the centrosome and plays an important role for successful mitosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Doxsey S . (2002). Mol Cell 10: 439–440.
Efimov VP, Morris NR . (2000). J Cell Biol 150: 681–688.
Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O'Connell CB, Wang Y et al. (2000). Nat Cell Biol 2: 784–791.
Feng Y, Olson EC, Stukenberg PT, Flanagan LA, Kirschner MW, Walsh CA . (2000). Neuron 28: 665–679.
Feng Y, Walsh CA . (2004). Neuron 44: 279–293.
Ghadimi BM, Sackett DL, Difilippantonio MJ, Schrock E, Neumann T, Jauho A et al. (2000). Genes Chromosomes Cancer 27: 183–190.
Howell BJ, Hoffman DB, Fang G, Murray AW, Salmon ED . (2000). J Cell Biol 150: 1233–1250.
Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED . (2004). Curr Biol 14: 953–964.
Lingle WL, Salisbury JL . (1999). Am J Pathol 155: 1941–1951.
Lingle WL, Salisbury JL . (2000). Curr Top Dev Biol 49: 313–329.
Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS et al. (2000). Neuron 28: 697–711.
Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P et al. (1998). Cancer Res 58: 3974–3985.
Pihan GA, Purohit A, Wallace J, Malhotra R, Liotta L, Doxsey SJ . (2001). Cancer Res 61: 2212–2219.
Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS . (2005). Science 307: 127–129.
Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A et al. (2000). Neuron 28: 681–696.
Sato N, Mizumoto K, Nakamura M, Nakamura K, Kusumoto M, Niiyama H et al. (1999). Clin Cancer Res 5: 963–970.
Shono M, Sato N, Mizumoto K, Maehara N, Nakamura M, Nagai E et al. (2001). Lab Invest 81: 945–952.
Wang Q, Hirohashi Y, Furuuchi K, Zhao H, Liu Q, Zhang H et al. (2004). DNA Cell Biol 23: 475–489.
Wang Q, Zhang H, Guerrette S, Chen J, Mazurek A, Wilson T et al. (2001). Oncogene 20: 4640–4649.
Wang Q, Zhang H, Kajino K, Greene MI . (1998). Oncogene 17: 1939–1948.
Yan X, Li F, Liang Y, Shen Y, Zhao X, Huang Q et al. (2003). Mol Cell Biol 23: 1239–1250.
Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey SJ . (2000). Mol Biol Cell 11: 2047–2056.
Acknowledgements
This work was supported by grants from the Abramson Family Cancer Research Institute of the University of Pennsylvania and from the National Institute of Health. We thank the Sir William Dunn School of Pathology, University of Oxford for providing the research facilities to conduct part of the studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Hirohashi, Y., Wang, Q., Liu, Q. et al. Centrosomal proteins Nde1 and Su48 form a complex regulated by phosphorylation. Oncogene 25, 6048–6055 (2006). https://doi.org/10.1038/sj.onc.1209637
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209637
Keywords
This article is cited by
-
NDE1 positively regulates oligodendrocyte morphological differentiation
Scientific Reports (2018)
-
Severe congenital microcephaly with 16p13.11 microdeletion combined with NDE1 mutation, a case report and literature review
BMC Medical Genetics (2017)
-
NDE1 and NDEL1 from genes to (mal)functions: parallel but distinct roles impacting on neurodevelopmental disorders and psychiatric illness
Cellular and Molecular Life Sciences (2017)
-
Regulation of the actin cytoskeleton by the Ndel1-Tara complex is critical for cell migration
Scientific Reports (2016)
-
The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies
Cellular and Molecular Life Sciences (2013)