Abstract
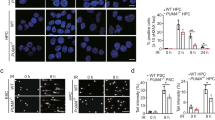
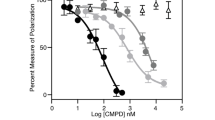
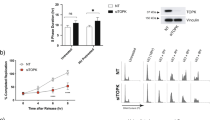
The retinoblastoma (pRB) family proteins regulate the E2F transcription factors; their complexes regulate critical transitions through the cell cycle. The function of these pRB family/E2F complexes, which includes p130/E2F4, in response to genotoxic agents, is not well understood. We investigated the role of E2F4 in the genotoxic stress response. Following radiation treatment, E2F4 colocalized with p130 in the nucleus during a radiation-induced stable G2-phase arrest. Arrested cells had significantly decreased expression of Cyclins A2 and B1 and decreased phosphorylation of mitotic protein monoclonal-2 (MPM-2) mitotic proteins. Small interference RNA (siRNA)-mediated knockdown of E2F4 sensitized cells to subsequent irradiation, resulting in enhanced cellular DNA damage and cell death, as determined by caspase activation and decreased clonogenic cell survival. Downstream E2F4 targets potentially involved in the progression from G2 into M phase were identified by oligonucleotide microarray expression profiling. Chromatin immunoprecipitation localized E2F4 at promoter regions of the Bub3 and Pttg1 mitotic genes following irradiation, which were among the downregulated genes identified by the microarray. These data suggest that in response to radiation, E2F4 becomes active in the nucleus, enforces a stable G2 arrest by target gene repression, and thus provides increased cell survival ability by minimizing propagation of cells that have irreparable DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Almasan A, Linke S, Paulson T, Huang L-c, Wahl GM . (1995a). Genetic instability as a consequence of inappropriate entry and progression through S-phase. Cancer Metast Rev 14: 59–73.
Almasan A, Yin Y, Kelly RE, Lee EY, Bradley A, Li W et al. (1995b). Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc Natl Acad Sci USA 92: 5436–5440.
Attwooll C, Denchi EL, Helin K . (2004). The E2F family: specific functions and overlapping interests. EMBO J 23: 4709–4716.
Baus F, Gire V, Fisher D, Piette J, Dulic V . (2003). Permanent cell cycle exit in G2 phase after DNA damage in normal human fibroblasts. EMBO J 22: 3992–4002.
Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ et al. (2005). Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res 65: 11597–11604.
Bindra RS, Glazer PM . (2005). Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res 569: 75–85.
Budde A, Schneiderhan-Marra N, Petersen G, Brune B . (2005). Retinoblastoma susceptibility gene product pRB activates hypoxia-inducible factor-1 (HIF-1). Oncogene 24: 1802–1808.
Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP et al. (1998). Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501.
Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B . (2000). Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev 14: 1584–1588.
Chang BD, Broude EV, Fang J, Kalinichenko TV, Abdryashitov R, Poole JC et al. (2000). p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene 19: 2165–2170.
Cobrinik D . (2005). Pocket proteins and cell cycle control. Oncogene 24: 2796–2809.
Crosby ME, Almasan A . (2004). Opposing roles of E2Fs in cell proliferation and death. Cancer Biol Ther 3: 1208–1211.
Crosby ME, Oancea M, Almasan A . (2004). p53 binding to target sites is dynamically regulated before and after ionizing radiation-mediated DNA damage. J Environ Pathol Toxicol Oncol 23: 67–79.
Dimova DK, Dyson NJ . (2005). The E2F transcriptional network: old acquaintances with new faces. Oncogene 24: 2810–2826.
DuPree EL, Mazumder S, Almasan A . (2004). Genotoxic stress induces expression of E2F4, leading to its association with p130 in prostate carcinoma cells. Cancer Res 64: 4390–4393.
Farkas T, Hansen K, Holm K, Lukas J, Bartek J . (2002). Distinct phosphorylation events regulate p130- and p107-mediated repression of E2F-4. J Biol Chem 277: 26741–26752.
Gaubatz S, Lees JA, Lindeman GJ, Livingston DM . (2001). E2F4 is exported from the nucleus in a CRM1-dependent manner. Mol Cell Biol 21: 1384–1392.
Giangrande PH, Zhu W, Schlisio S, Sun X, Mori S, Gaubatz S et al. (2004). A role for 2A7E in distinguishing G1/S- and G2/M-specific transcription. Genes Dev 18: 2941–2951.
Herrera RE, Sah VP, Williams BO, Makela TP, Weinberg RA, Jacks T . (1996). Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol 16: 2402–2407.
Iliakis G, Wang Y, Guan J, Wang H . (2003). DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 22: 5834–5847.
Innocente SA, Abrahamson JL, Cogswell JP, Lee JM . (1999). p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA 96: 2147–2152.
Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M et al. (2001). Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol 21: 4684–4699.
Jackson MW, Agarwal MK, Yang J, Bruss P, Uchiumi T, Agarwal ML et al. (2005). p130/p107/p105Rb-dependent transcriptional repression during DNA-damage-induced cell-cycle exit at G2 . J Cell Sci 118: 1821–1832.
Kastan MB, Bartek J . (2004). Cell-cycle checkpoints and cancer. Nature 432: 316–323.
Linke SP, Clarkin KC, Di Leonardo A, Tsou A, Wahl GM . (1996). A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev 10: 934–947.
Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL et al. (2003). Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113: 703–716.
Niculescu III AB, Chen X, Smeets M, Hengst L, Prives C, Reed SI . (1998). Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol 18: 629–643.
Ossovskaya VS, Mazo IA, Chernov MV, Chernova OB, Strezoska Z, Kondratov R et al. (1996). Use of genetic suppressor elements to dissect distinct biological effects of separate p53 domains. Proc Natl Acad Sci USA 93: 10309–10314.
Pickering MT, Kowalik TF . (2006). Rb inactivation leads to E2F1-mediated DNA double-strand break accumulation. Oncogene 25: 746–755.
Polager S, Ginsberg D . (2003). E2F mediates sustained G2 arrest and down-regulation of Stathmin and AIM-1 expression in response to genotoxic stress. J Biol Chem 278: 1443–1449.
Ray S, Almasan A . (2003). Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res 63: 4713–4723.
Rayman JB, Takahashi Y, Indjeian VB, Dannenberg JH, Catchpole S, Watson RJ et al. (2002). E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev 16: 933–947.
Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA et al. (2002). E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev 16: 245–256.
Rogoff HA, Pickering MT, Debatis ME, Jones S, Kowalik TF . (2002). E2F1 induces phosphorylation of p53 that is coincident with p53 accumulation and apoptosis. Mol Cell Biol 22: 5308–5318.
Sardet C, Vidal M, Cobrinik D, Geng Y, Onufryk C, Chen A et al. (1995). E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci USA 92: 2403–2407.
Stevens C, Smith L, La Thangue NB . (2003). Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol 5: 401–409.
Takahashi Y, Rayman JB, Dynlacht BD . (2000). Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev 14: 804–816.
Taylor WR, Schonthal AH, Galante J, Stark GR . (2001). p130/E2F4 binds to and represses the cdc2 promoter in response to p53. J Biol Chem 276: 1998–2006.
Taylor WR, Stark GR . (2001). Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815.
Trimarchi JM, Lees JA . (2002). Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3: 11–20.
Waghray A, Schober M, Feroze F, Yao F, Virgin J, Chen YQ . (2001). Identification of differentially expressed genes by serial analysis of gene expression in human prostate cancer. Cancer Res 61: 4283–4286.
Wahl GM, Carr AM . (2001). The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol 3: E277–E286.
Wells J, Boyd KE, Fry CJ, Bartley SM, Farnham PJ . (2000). Target gene specificity of E2F and pocket protein family members in living cells. Mol Cell Biol 20: 5797–5807.
Xu B, Kim ST, Lim DS, Kastan MB . (2002). Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol Cell Biol 22: 1049–1059.
Yan T, Desai AB, Jacobberger JW, Sramkoski RM, Loh T, Kinsella TJ . (2004). CHK1 and CHK2 are differentially involved in mismatch repair-mediated 6-thioguanine-induced cell cycle checkpoint responses. Mol Cancer Ther 3: 1147–1157.
Zhu W, Giangrande PH, Nevins JR . (2004). E2Fs link the control of G1/S and G2/M transcription. Embo J 23: 4615–4626.
Acknowledgements
We thank Drs W Heston and A Gudkov (Cleveland Clinic) for valuable reagents and B Dynlacht (NYU) for advice. We also thank the Flow Cytometry and Imaging Core facilities staff at the Cleveland Clinic Foundation, and RM Sramkowski at the Case Comprehensive Cancer Center for their invaluable help. This work was supported by research grants from the US National Institute of Health (CA81504 and CA82858).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Crosby, M., Jacobberger, J., Gupta, D. et al. E2F4 regulates a stable G2 arrest response to genotoxic stress in prostate carcinoma. Oncogene 26, 1897–1909 (2007). https://doi.org/10.1038/sj.onc.1209998
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209998