Abstract
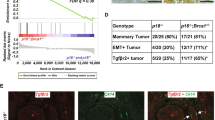
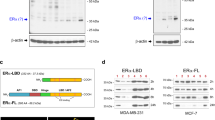
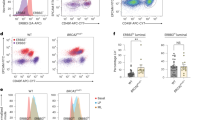
Estrogen and its receptor alpha (ERα) have been implicated in the tissue-specific tumorigenesis associated with BRCA1 mutations. However, the majority of breast cancers developed in human BRCA1 mutation carriers are ERα-negative, challenging the link between BRCA1 and estrogen/ERα in breast cancer formation. Using a mouse model lacking the full-length form of BRCA1, here we show that ERα is highly expressed in the premalignant mammary gland and initiation stages of tumorigenesis, although its expression is gradually diminished during mammary tumor progression. We demonstrate that the absence of full-length BRCA1 increases sensitivity of cells to estrogen-induced extracellular signal-regulated kinase 1/2 phosphorylation and cyclin D1 expression. The absence of BRCA1 turns the proliferation of ERα-positive cells from a paracrine fashion to an autocrine or endocrine fashion. Consequently, BRCA1-mutant cells are sensitized to estrogen-induced cell proliferation in vitro and mammary tumorigenesis in vivo. These findings illustrate a molecular mechanism for estrogen/ERα signals in BRCA1-associated tissue-specific tumor formation, and identify several key elements in the estrogen/ERα-signaling cascade that can serve as potential therapeutic targets for BRCA1-associated tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Alberg AJ, Lam AP, Helzlsouer KJ . (1999). Epidemiology, prevention, and early detection of breast cancer. Curr Opin Oncol 11: 435–441.
Bachelier R, Li C, Qiao W, Furth PA, Lubet RA, Deng CX . (2005). Effects of bilateral oophorectomy on mammary tumor formation in breast cancer associated gene 1 (Brca1) mutant mice. Oncol Rep 14: 1117–1120.
Brodie SG, Xu X, Qiao W, Li WM, Cao L, Deng CX . (2001). Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene 20: 7514–7523.
Calderon-Margalit R, Paltiel O . (2004). Prevention of breast cancer in women who carry BRCA1 or BRCA2 mutations: a critical review of the literature. Int J Cancer 112: 357–364.
Chodankar R, Kwang S, Sangiorgi F, Hong H, Yen HY, Deng C et al. (2005). Cell-nonautonomous induction of ovarian and uterine serous cystadenomas in mice lacking a functional Brca1 in ovarian granulosa cells. Curr Biol 15: 561–565.
Clarke RB, Howell A, Potten CS, Anderson E . (1997). Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57: 4987–4991.
Deng CX, Wang RH . (2003). Roles of BRCA1 in DNA damage repair: a link between development and cancer. Hum Mol Genet 12: R113–R123.
Deng CX, Xu X . (2004). Generation and analysis of Brca1 conditional knockout mice. Methods Mol Biol 280: 185–200.
Deng CX . (2006). BRCA1: cell cycle checkpoint, genetic instability, DNA damage response, and cancer evolution. Nucleic Acids Res 34: 1416–1426.
Eisinger F, Jacquemier J, Nogues C, Birnbaum D, Sobol H . (1999). Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer 85: 2291–2295.
Elledge SJ, Amon A . (2002). The BRCA1 suppressor hypothesis: an explanation for the tissue-specific tumor development in BRCA1 patients. Cancer Cell 1: 129–132.
Fan S, Wang J, Yuan R, Ma Y, Meng Q, Erdos MR et al. (1999). BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 284: 1354–1356.
Gadducci A, Biglia N, Sismondi P, Genazzani AR . (2005). Breast cancer and sex steroids: critical review of epidemiological, experimental and clinical investigations on etiopathogenesis, chemoprevention and endocrine treatment of breast cancer. Gynecol Endocrinol 20: 343–360.
Hu Y, Ghosh S, Amleh A, Yue W, Lu Y, Katz A et al. (2005). Modulation of aromatase expression by BRCA1: a possible link to tissue-specific tumor suppression. Oncogene 24: 8343–8348.
Huo Z, Giger ML, Olopade OI, Wolverton DE, Weber BL, Metz CE et al. (2002). Computerized analysis of digitized mammograms of BRCA1 and BRCA2 gene mutation carriers. Radiology 225: 519–526.
Johannsson OT, Idvall I, Anderson C, Borg A, Barkardottir RB, Egilsson V et al. (1997). Tumour biological features of BRCA1-induced breast and ovarian cancer. Eur J Cancer 33: 362–371.
Karp SE, Tonin PN, Begin LR, Martinez JJ, Zhang JC, Pollak MN et al. (1997). Influence of BRCA1 mutations on nuclear grade and estrogen receptor status of breast carcinoma in Ashkenazi Jewish women. Cancer 80: 435–441.
Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA et al. (2002). Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346: 1609–1615.
Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS et al. (1996). Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res 51: 159–186; discussion 186–188.
Lane TF, Deng C, Elson A, Lyu MS, Kozak CA, Leder P . (1995). Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev 9: 2712–2722.
Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E et al. (1996). Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 15: 1292–1300.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S et al. (1994). A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266: 66–71.
Monteiro AN . (2003). BRCA1: the enigma of tissue-specific tumor development. Trends Genet 19: 312–315.
Prall OW, Rogan EM, Sutherland RL . (1998). Estrogen regulation of cell cycle progression in breast cancer cells. J Steroid Biochem Mol Biol 65: 169–174.
Russo IH, Russo J . (1998). Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia 3: 49–61.
Russo J, Ao X, Grill C, Russo IH . (1999). Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat 53: 217–227.
Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH et al. (2002). The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol 80: 239–256.
Trauernicht AM, Boyer TG . (2003). BRCA1 and estrogen signaling in breast cancer. Breast Dis 18: 11–20.
Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV et al. (2004). BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14: 2135–2142.
Venkitaraman AR . (2002). Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108: 171–182.
Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA et al. (2001). Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet 28: 266–271.
Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T et al. (1999). Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation [see comments]. Nat Genet 22: 37–43.
Zhang J, Powell SN . (2005). The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res 3: 531–539.
Zheng L, Annab LA, Afshari CA, Lee WH, Boyer TG . (2001). BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci USA 98: 9587–9592.
Acknowledgements
We thank Thomas Fishler, Yohei Tominaga and Rui-Hong Wang for critical reading of this paper. This research was supported by the Intramural Research Program of the National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Xiao, C., Vonderhaar, B. et al. A role of estrogen/ERα signaling in BRCA1-associated tissue-specific tumor formation. Oncogene 26, 7204–7212 (2007). https://doi.org/10.1038/sj.onc.1210527
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210527
Keywords
This article is cited by
-
Effects of obesity on breast aromatase expression and systemic metabo-inflammation in women with BRCA1 or BRCA2 mutations
npj Breast Cancer (2021)
-
NOTCH1 activation compensates BRCA1 deficiency and promotes triple-negative breast cancer formation
Nature Communications (2020)
-
Estrogen promotes estrogen receptor negative BRCA1-deficient tumor initiation and progression
Breast Cancer Research (2018)
-
Mouse models of BRCA1 and their application to breast cancer research
Cancer and Metastasis Reviews (2013)
-
BRCA1 promoter methylation status does not predict response to tamoxifen in sporadic breast cancer patients
Breast Cancer Research and Treatment (2012)