Abstract
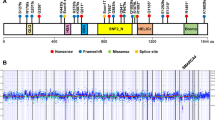
Endometrial carcinoma is the most common gynecological malignancy in the United States. Although most women present with early disease confined to the uterus, the majority of persistent or recurrent tumors are refractory to current chemotherapies. We have identified a total of 11 different FGFR2 mutations in 3/10 (30%) of endometrial cell lines and 19/187 (10%) of primary uterine tumors. Mutations were seen primarily in tumors of the endometrioid histologic subtype (18/115 cases investigated, 16%). The majority of the somatic mutations identified were identical to germline activating mutations in FGFR2 and FGFR3 that cause Apert Syndrome, Beare–Stevenson Syndrome, hypochondroplasia, achondroplasia and SADDAN syndrome. The two most common somatic mutations identified were S252W (in eight tumors) and N550K (in five samples). Four novel mutations were identified, three of which are also likely to result in receptor gain-of-function. Extensive functional analyses have already been performed on many of these mutations, demonstrating they result in receptor activation through a variety of mechanisms. The discovery of activating FGFR2 mutations in endometrial carcinoma raises the possibility of employing anti-FGFR molecularly targeted therapies in patients with advanced or recurrent endometrial carcinoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Burgar HR, Burns HD, Elsden JL, Lalioti MD, Heath JK . (2002). Association of the signaling adaptor FRS2 with fibroblast growth factor receptor 1 (Fgfr1) is mediated by alternative splicing of the juxtamembrane domain. J Biol Chem 277: 4018–4023.
Burset M, Seledtsov IA, Solovyev VV . (2000). Analysis of canonical and non-canonical splice sites in mammalian genomes. Nucleic Acids Res 28: 4364–4375.
Cho JY, Guo C, Torello M, Lunstrum GP, Iwata T, Deng C et al. (2004). Defective lysosomal targeting of activated fibroblast growth factor receptor 3 in achondroplasia. Proc Natl Acad Sci USA 101: 609–614.
Chong A, Zhang G, Bajic VB . (2004). Information for the Coordinates of Exons (ICE): a human splice sites database. Genomics 84: 762–766.
Gold LI, Saxena B, Mittal KR, Marmor M, Goswami S, Nactigal L et al. (1994). Increased expression of transforming growth factor beta isoforms and basic fibroblast growth factor in complex hyperplasia and adenocarcinoma of the endometrium: evidence for paracrine and autocrine action. Cancer Res 54: 2347–2358.
Greenman C, Stephens P, Smith R, Dalgliesh G, Hunter C, Bignell G et al. (2007). Patterns of Somatic mutation in cancer genomes. Nature 446: 153–158.
Ibrahimi OA, Eliseenkova AV, Plotnikov AN, Yu K, Ornitz DM, Mohammadi M . (2001). Structural basis for fibroblast growth factor receptor 2 activation in Apert syndrome. Proc Natl Acad Sci USA 98: 7182–7187.
Ibrahimi OA, Zhang F, Eliseenkova AV, Itoh N, Linhardt RJ, Mohammadi M . (2004). Biochemical analysis of pathogenic ligand-dependent FGFR2 mutations suggests distinct pathophysiological mechanisms for craniofacial and limb abnormalities. Hum Mol Genet 13: 2313–2324.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C : et al. (2006). Cancer statistics, 2006. CA Cancer J Clin 56: 106–130.
Mohammadi M, Olsen SK, Ibrahimi OA . (2005). Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 16: 107–137.
Moller B, Rasmussen C, Lindblom B, Olovsson M . (2001). Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reprod 7: 65–72.
Monsonego-Ornan E, Adar R, Feferman T, Segev O, Yayon A . (2000). The transmembrane mutation G380R in fibroblast growth factor receptor 3 uncouples ligand-mediated receptor activation from down-regulation. Mol Cell Biol 20: 516–522.
Obel JC, Friberg G, Fleming GF . (2006). Chemotherapy in endometrial cancer. Clin Adv Hematol Oncol 4: 459–468.
Ornitz DM, Itoh N . (2001). Fibroblast growth factors. Genome Biol 2: Reviews3005.1–reviews3005.12.
Passos-Bueno MR, Wilcox WR, Jabs EW, Sertie AL, Alonso LG, Kitoh H . (1999). Clinical spectrum of fibroblast growth factor receptor mutations. Hum Mutat 14: 115–125.
Sangha RK, Li XF, Shams M, Ahmed A . (1997). Fibroblast growth factor receptor-1 is a critical component for endometrial remodeling: localization and expression of basic fibroblast growth factor and FGF-R1 in human endometrium during the menstrual cycle and decreased FGF-R1 expression in menorrhagia. Lab Invest 77: 389–402.
Tsai SJ, Wu MH, Chen HM, Chuang PC, Wing LY . (2002). Fibroblast growth factor-9 is an endometrial stromal growth factor. Endocrinology 143: 2715–2721.
van Rhijn BW, Lurkin I, Radvanyi F, Kirkels WJ, van der Kwast TH, Zwarthoff EC . (2001). The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res 61: 1265–1268.
Wilkie AO . (2005). Bad bones, absent smell, selfish testes: the pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev 16: 187–203.
Wilkie AO, Patey SJ, Kan SH, van den Ouweland AM, Hamel BC . (2002). FGFs, their receptors, and human limb malformations: clinical and molecular correlations. Am J Med Genet 112: 266–278.
Yu K, Herr AB, Waksman G, Ornitz DM . (2000). Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc Natl Acad Sci USA 97: 14536–14541.
Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM . (2006). Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 281: 15694–15700.
Acknowledgements
We thank the TGen Sequencing Core for their excellent work. We also thank Feng Gao and the Biostatistics Core at Siteman Cancer Center, Barnes-Jewish Hospital Washington University for assistance with the survival analyses (CA091842). Supported in part by RO1 CA71754 (PJG and MAM), Wellcome Trust (MS, AF and HD), the Melanoma Research Foundation (PMP) and R01 CA109544 (JT).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Pollock, P., Gartside, M., Dejeza, L. et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene 26, 7158–7162 (2007). https://doi.org/10.1038/sj.onc.1210529
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210529
Keywords
This article is cited by
-
FGFR-targeted therapeutics: clinical activity, mechanisms of resistance and new directions
Nature Reviews Clinical Oncology (2024)
-
Efficacy of futibatinib, an irreversible fibroblast growth factor receptor inhibitor, in FGFR-altered breast cancer
Scientific Reports (2023)
-
Oncogenic Y68 frame shift mutation of PTEN represents a mechanism of docetaxel resistance in endometrial cancer cell lines
Scientific Reports (2019)
-
Nuclear Fibroblast Growth Factor Receptor Signaling in Skeletal Development and Disease
Current Osteoporosis Reports (2019)
-
A homologous mapping method for three-dimensional reconstruction of protein networks reveals disease-associated mutations
BMC Systems Biology (2018)