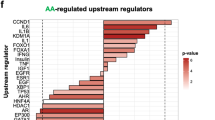
Abstract
α1-Adrenoceptor antagonists, have been documented to induce apoptosis and reduce prostate tumor vascularity in benign and malignant prostate cells. The quinazoline based α1-antagonists, doxazosin and terazosin but not tamsulosin (a sulphonamide derivative) suppress prostate growth without affecting cell proliferation. These quinazoline-mediated apoptotic effects occur via an α1-adrenoceptor independent mechanism potentially involving activation of the TGF-β signal transduction pathway. This review discusses the current knowledge of the action of quinazoline-derived α1-adrenoceptor antagonists in the benign and malignant prostate and their potential therapeutic use in the treatment of benign prostatic hyperplasia (BPH) and prostate cancer. Finally, a molecular pathway is proposed for their observed apoptotic function against prostate cells. Increased understanding of the action of these established and clinically accepted agents would provide a basis for the design of safe, effective therapeutic regimens in the treatment of prostatic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Greenlee RT, Murray T, Bolden S, Wingo PA . Cancer statistics, 2000 CA Cancer J Clin 2000 50: 7–33
Boyle P . Prostate Cancer 2000; evolution of an epidemic of unknown origin In: Dennis L (ed) Prostate Cancer 2000 Springer: Heidelberg 1994 pp 5–11
Isaacs JT . Role of androgens in prostatic cancer Vit and Horm 1994 49: 433–502
Catalona WJ, Smith DS, Ratliff TL, Basler JW . Detection of organ confined prostate cancer is associated through prostate-specific antigen-based screening JAMA 1993 270: 948–954
Richie JP et al. Effect of patient age on early detection of prostate cancer with prostate specific antigen and digital rectal examination Urology 1993 42: 365–374
Raghavan D . Non-hormone chemotherapy for prostate cancer: principles of treatment and application to the testing of new drugs Semin Oncol 1988 15: 371–389
Crawford ED et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma New Engl J Med 1989 321: 419–424
Zelefsky MJ et al. Neoadjuvant hormonal therapy improves the therapeutic ration in patients with bulky prostatic disease treated with three dimensional conformal therapy Int J Rad Oncol Biol Phys 1994 29: 755–761
Coffey DS, Walsh PC . Clinical and experimental studies of benign prostatic hyperplasia Urol Clin North Am 1990 17: 461–475
Kyprianou N . Role of apoptosis in development of benign prostatic hyperplasia (BPH) and prostate cancer: clinical significance in diagnosis and treatment In: Naz RN (ed) Prostate: Basic and Clinical Aspects CRC Press: Inc Boca Raton, FL 1997 pp 181–199
Bruckheimer EM, Kyprianou N . Apoptosis in prostate carcinogenesis. A growth regulator and a therapeutic target Cell Tissue Res 2000 301: 153–162
Kyprianou N, Isaacs JT . Activation of programmed cell death in the rat ventral prostate after castration Endocrinology 1988 122: 552–562
Kyprianou N, English HF, Isaacs JT . Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation Cancer Res 1990 50: 3748–3753
Kyprianou N, Bains AK, Jacobs SC . Induction of apoptosis in androgen-independent human prostate cancer cells undergoing thymineless death Prostate 1994 25: 66–75
Sklar GN, Eddy HA, Jacobs SC, Kyprianou N . Combined antitumor effect of suramin plus irradiation in human prostate cancer cells: the role of apoptosis J Urol 1993 150: 1526–1532
Furuya Y, Krajewski S, Epstein JI, Isaacs JT . Expression of bcl-2 and the progression of human and rodent prostate cancers Clin Cancer Res 1996 2: 389–398
Rupnow BA et al. Direct evidence that apoptosis enhances tumor responses to fractionated radiotherapy Cancer Res 1998 58: 1779–1784
Caine M, Raz S, Zeigler M . Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck Br J Urol 1975 47: 193–202
Lepor H, Shapiro E . Characterization of the α1-adrenergic receptors in human benign prostatic hyperplasia J Urol 1984 132: 396–398
Walden PD et al. Localization of mRNA and receptor binding sites for the α1 a-adrenoceptor subtype in the rat, monkey and human urinary bladder and prostate J Urol 1997 157: 1032–1038
Caine M . Alpha-adrenergic mechanisms in dynamics of benign prostatic hypertrophy Urology 1988 32: 16–20
Caine M, Pfau A, Pelberg S . The use of alpha-adrenergic blockers in benign prostatic hyperplasia Br J Urol 1976 48: 255–263
Lepor H, Tang R, Shapiro E . The alpha-adrenoreceptor subtype mediating the tension of human prostatic smooth muscle Prostate 1993 22: 301–307
Chapple CR et al. Alpha 1-adrenoreceptor subtypes in the human prostate Br J Urol 1994 74: 585–589
Beduschi MC, Beduschi R, Oesterling JE . Alpha-blockade therapy for benign prostatic hyperplasia: from a nonselective to a more selective alpha1 a-adrenergic antagonist Urology 1998 51: 861–872
Andersson KE, Lepor H, Wyllie MG . Prostatic alpha 1-adrenoreceptors and uroselectivity Prostate 1997 30: 202–215
Smith P, Rhodes NP, Ke Y, Foster CS . Influence of the α1-adrenergic antagonist, doxazosin, on noradrenalin-induced modulation of cytoskeletal proteins in cultured hyperplastic prostatic stromal cells Prostate 1999 38: 216–227
Kirby RS . Doxazosin in treatment of the lower urinary tract In: Kirby RS, McConnel JD, Fitzpatrick JM, Roeherborn CG, Boyle P (eds) Textbook of Benign Prostatic Hyperplasia ISIS Medical Media: Oxford 1996 pp 287–293
Young RA, Brogden RM . Doxazosin: a review of its pharmacodynamic and pharmokinetic properties, and therapeutic efficacy in mild or moderate hypertension Drugs 1988 33: 525–541
Sonders RC . Pharmacokinetics of terazosin Am J Med 1986 80: 20–24
Kyprianou N et al. Induction of prostatic apoptosis by doxazosin in benign prostatic hyperplasia J Urol 1998 159: 1810–1815
Chon JK et al. α1-Adrenoreceptor antagonists terazosin and doxazosin induce prostate apoptosis without affecting all proliferation in patients with benign prostatic hyperplasia J Urol 1999 161: 2002–2008
Kyprianou N, Benning CM . Suppression of human prostate cancer cell growth by alpha 1-adrenoreceptor antagonists doxazosin and terazosin via induction of apoptosis Cancer Res 2000 60: 4550–4555
Yang G et al. Transforming growth factor-β1 transduced mouse prostate reconstitutions: II. Induction of apoptosis by doxazosin Prostate 1997 33: 157–163
Cal C et al. Doxazosin. A new cytotoxic agent for prostate cancer? BJU Intl 2000 85: 672–675
Kyprianou N, Chon J, Benning CM . Effects of alpha(1)-adrenoceptor (alpha(1)-AR) antagonists on cell proliferation and apoptosis in the prostate: therapeutic implications in prostatic disease Prostate 2000 9: (Suppl) 42–46
Wrana JL et al. Mechanism of activation of the TGF-beta receptor Nature 1994 370: 341–347
Kyprianou N, Isaacs JT . Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death Mol Endocrinol 1989 3: 1515–1522
Martikainen P, Kyprianou N, Isaacs JT . Effect of transforming growth factor-β1 on proliferation and death of rat prostatic cells Endocrinology 1990 127: 2963–2968
Kyprianou N, Isaacs JT . Identification of a cellular receptor for transforming growth factor-β in rat ventral prostate and its negative regulation by androgens Endocrinology 1988 123: 2124–2131
Tu H, Borkowski A, Jacobs SC, Kyprianou N . Incidence of apoptosis and cell proliferation in prostate cancer: relationship with TGF-β and bcl-2 expression Int J Cancer 1996 69: 357–363
Anglin IE, Kyprianou N . Unpublished observations
Lehmann K et al. Raf induces TGF-β production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells Genes Dev 2000 14: 2610–2622
Lin HY et al. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase Cell 1992 68: 775–785
deCaestecker MP, Piek E, Roberts AB . Role of transforming growth factor-beta signaling in cancer J Natl Cancer Inst 2000 92: 1388–1402
Massague J . How cells read TGF-beta signals Nat Rev Mol Cell Biol 2000 1: 169–178
Massague J, Blain SW, Lo RS . TGF-beta signaling in growth control, cancer, and heritable disorders Cell 2000 103: 295–309
Brodin G et al. Increased Smad expression and activation are associated with apoptosis in normal and malignant prostate after castration Cancer Res 1999 59: 2731–2738
Kim IY et al. Loss of expression of transforming growth factor beta type I and type II receptors correlates with tumor grade in human prostate cancer tissues Clin Cancer Res 1996 2: 1255–1261
Williams RH et al. Reduced levels of transforming growth factor beta receptor type II in human prostate cancer: an immunohistochemical study Clin Cancer Res 1996 2: 635–640
Guo Y, Jacobs SC, Kyprianou N . Down-regulation of protein and mRNA expression for transforming growth factor-β (TGF-β1) Type I and Type II receptors in human prostate cancer Int J Cancer 1997 71: 573–579
Guo Y, Kyprianou N . Overexpression of transforming growth factor (TGF) beta l type II receptor restores TGF-beta l sensitivity and signaling in human prostate cancer cells Cell Growth Differ 1998 9: 185–193
Tsukazaki T et al. SARA, a FYVE domain protein that recruits Smad2 to the TGF beta receptor Cell 1998 95: 779–791
Liu F, Pouponnot C, Massague J . Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes Genes Dev 1997 11: 3157–3167
Kim SK et al. DPC4, a candidate tumor suppressor gene, is altered infrequently in head and neck squamous cell carcinoma Cancer Res 1996 56: 2519–2521
Muro-Cacho CA, Rosario-Ortiz K, Livingston S, Munoz-Antonia T . Defective transforming growth factor beta signaling pathway in head and neck squamous cell carcinoma as evidenced by the lack of expression of activated Smad2 Clin Cancer Res 2001 7: 1618–1626
Glassman DT et al. Combined effect of terazosin and finasteride on apoptosis, cell proliferation and transforming growth factor-β expression in benign prostatic hyperplasia Prostate 2001 46: 45–51
Chen W, Frank ME, Jin W, Wahl SM . TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu Immunity 2001 14: 715–725
Fabregat I et al. Epidermal growth factor impairs the cytochrome C/caspase-3 apoptotic pathway induced by transforming growth factor beta in rat fetal hepatocytes via a phosphoinositide 3-kinase-dependent pathway Hepatology 2000 32: 528–535
Chen RH, Su YH, Chuang RL, Chang TY . Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway Oncogene 1998 17: 1959–1968
Lin HK, Yeh S, Kang HY, Chang C . Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor Proc Natl Acad Sci USA 2001 98: 7200–7205
Grasso AW et al. ErbB kinases and NDF signaling in human prostate cancer cells Oncogene 1997 15: 2705–2716
Baldwin AS Jr . The NF-κB and IκB Proteins: New discoveries and insights Ann Rev Immunol 1996 14: 649–681
Ibrahim GK et al. Differential immunoreactivity of Her-2/neu oncoprotein in prostatic tissues Surg Oncol 1992 1: 151–155
Harper ME et al. Expression of androgen receptor and growth factors in premalignant lesions of the prostate J Pathol 1998 186: 169–177
Schwartz S Jr et al. Gains of the relative genomic content of erbB-1 and erbB-2 in prostate carcinoma and their association with metastasis Int J Oncol 1999 14: 367–371
Kretzschmar M, Doody J, Timokhina I, Massague J . A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras Genes Dev 1999 13: 804–816
Lo RS, Wotton D, Massague J . Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF EMBO J 2001 20: 128–136
Habib AA et al. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosome J Biol Chem 2001 276: 8865–8874
Yeh S et al. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells J Biol Chem 1999 96: 5458–5463
Craft N, Shostak Y, Carey M, Sawyers CL . A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signalling by the HER2/neu tyrosine kinase Nature Medicine 1999 5: 280–285
Wen Y et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway Cancer Res 2000 60: 6841–6845
del Peso L et al. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt Science 1997 278: 687–689
Bitzer M et al. A mechanism of suppression of TGF-β/SMAD signaling by NF-κB/Rel A Genes Dev 2000 14: 187–197
Baeuerle PA, Henkel T . Function and activation of NF-kappa B in the immune system Annu Rev Immunol 1994 12: 141–179
Karin M . How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex Oncogene 1999 18: 6867–6874
Patil S et al. Smad7 is induced by CD40 and protects WEHI 231 B-lymphocytes from transforming growth factor-beta-induced growth inhibition and apoptosis J Biol Chem 2000 275: 38363–38370
Sovak MA et al. The inhibitory effects of transforming growth factor-β1 on breast cancer cell proliferation are mediated through regulation of aberrant nuclear factor-κB/Rel expression Cell Growth Differentiation 1999 10: 537–544
Erl W et al. Nuclear Factor-κB regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells Circ Res 1999 84: 669–677
Anglin IE, Kyprianou N . Quinazoline based α1-adrenoceptor antagonists activate prostate cancer cell apoptosis via TGF-β signaling and caspase-3 activation Manuscript submitted for publication 2001
Arteaga CL, Ramsey TT, Shawver LK, Guyer CA . Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site J Biol Chem 1997 12: 272 23247–23254
Ciardiello F et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor Clin Cancer Res 2001 7: 1459–1465
Bruns CJ et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma Cancer Res 2000 60: 2926–2935
Keledjian K et al. Reduction of human prostate tumor vascularity by α1-adrenoreceptor antagonist terazosin Prostate 2001 48: 71–78
Tsou H-R et al. 6-Substituted-4-(3-bromophenylamino) quinazolines as putative irreversible inhibitors of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER-2) tyrosine kinases with enhanced antitumor activity J Med Chem 2001 44: 2719–2734
Benning C, Kyprianou N . Quinazoline-derived α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an α1-adrenoceptor-independent action Cancer Res 2002 (in press)
Rewcastle GW et al. Tyrosine kinase inhibitors. 5. Synthesis and structure-activity relationships for 4-[(phenylmethyl)amino]- and 4-(phenylamino) quinazolines as potent adenosine 5′-triphosphate binding site inhibitors of the tyrosine kinase domain of the epidermal growth factor receptor J Med Chem 1995 38: 3482–3487
Acknowledgements
These studies were supported by educational grants from Pfizer Pharmaceuticals and Abbot International.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anglin, I., Glassman, D. & Kyprianou, N. Induction of prostate apoptosis by α1-adrenoceptor antagonists: mechanistic significance of the quinazoline component. Prostate Cancer Prostatic Dis 5, 88–95 (2002). https://doi.org/10.1038/sj.pcan.4500561
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500561
Keywords
This article is cited by
-
Role of α- and β-adrenergic signaling in phenotypic targeting: significance in benign and malignant urologic disease
Cell Communication and Signaling (2021)
-
A Pilot retrospective analysis of alpha-blockers on recurrence in men with localised prostate cancer treated with radiotherapy
Scientific Reports (2020)
-
New intraprostatic injectables and prostatic urethral lift for male LUTS
Nature Reviews Urology (2015)
-
Pharmacological characterization of N1-(2-methoxyphenyl)-N4-hexylpiperazine as a multi-target antagonist of α1A/α1D-adrenoceptors and 5-HT1A receptors that blocks prostate contraction and cell growth
Naunyn-Schmiedeberg's Archives of Pharmacology (2014)
-
RETRACTED ARTICLE: Observational study: daily treatment with a new compound “tradamixina” plus serenoa repens for two months improved the lower urinary tract symptoms
BMC Surgery (2012)