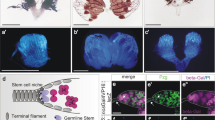
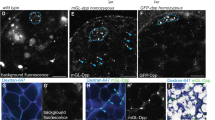
Abstract
Adult stem cells reside in specialized microenvironments, or niches, that have an important role in regulating stem cell behaviour1. Therefore, tight control of niche number, size and function is necessary to ensure the proper balance between stem cells and progenitor cells available for tissue homeostasis and wound repair. The stem cell niche in the Drosophila male gonad is located at the tip of the testis where germline and somatic stem cells surround the apical hub, a cluster of approximately 10–15 somatic cells that is required for stem cell self-renewal and maintenance2,3,4. Here we show that somatic stem cells in the Drosophila testis contribute to both the apical hub and the somatic cyst cell lineage. The Drosophila orthologue of epithelial cadherin (DE-cadherin) is required for somatic stem cell maintenance and, consequently, the apical hub. Furthermore, our data indicate that the transcriptional repressor escargot regulates the ability of somatic cells to assume and/or maintain hub cell identity. These data highlight the dynamic relationship between stem cells and the niche and provide insight into genetic programmes that regulate niche size and function to support normal tissue homeostasis and organ regeneration throughout life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978)
Hardy, R. W., Tokuyasu, K. T., Lindsley, D. L. & Garavito, M. The germinal proliferation center in the testis of Drosophila melanogaster . J. Ultrastruct. Res. 69, 180–190 (1979)
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001)
Tulina, N. & Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546–2549 (2001)
Jones, D. L. & Wagers, A. J. No place like home: anatomy and function of the stem cell niche. Nature Rev. Mol. Cell. Biol. 9, 11–21 (2008)
Kiger, A. A., White-Cooper, H. & Fuller, M. T. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407, 750–754 (2000)
Tran, J., Brenner, T. J. & DiNardo, S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature 407, 754–757 (2000)
Harrison, D. A., McCoon, P. E., Binari, R., Gilman, M. & Perrimon, N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 12, 3252–3263 (1998)
Gönczy, P. & DiNardo, S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122, 2437–2447 (1996)
Wallenfang, M. R., Nayak, R. & DiNardo, S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster . Aging Cell 5, 297–304 (2006)
Boyle, M., Wong, C., Rocha, M. & Jones, D. L. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1, 470–478 (2007)
Boyle, M. & DiNardo, S. Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development 121, 1815–1825 (1995)
Harrison, D. A. & Perrimon, N. Simple and efficient generation of marked clones in Drosophila . Curr. Biol. 3, 424–433 (1993)
Gönczy, P., Viswanathan, S. & DiNardo, S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development 114, 89–98 (1992)
Yamashita, Y., Jones, D. L. & Fuller, M. T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550 (2003)
Li, M. A., Alls, J. D., Avancini, R. M., Koo, K. & Godt, D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila . Nature Cell Biol. 5, 994–1000 (2003)
Schulz, C., Wood, C. G., Jones, D. L., Tazuke, S. I. & Fuller, M. T. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523–4534 (2002)
Song, X., Zhu, C. H., Doan, C. & Xie, T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855–1857 (2002)
Song, X. & Xie, T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl Acad. Sci. USA 99, 14813–14818 (2002)
Streit, A., Bernasconi, L., Sergeev, P., Cruz, A. & Steinmann-Zwicky, M. mgm 1, the earliest sex-specific germline marker in Drosophila, reflects expression of the gene esg in male stem cells. Int. J. Dev. Biol. 46, 159–166 (2002)
Le Bras, S. & Van Doren, M. Development of the male germline stem cell niche in Drosophila . Dev. Biol. 294, 92–103 (2006)
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005)
Adams, G. B. et al. Therapeutic targeting of a stem cell niche. Nature Biotechnol. 25, 238–243 (2007)
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005)
Sneddon, J. B. et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc. Natl Acad. Sci. USA 103, 14842–14847 (2006)
Nystul, T. & Spradling, A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1, 277–285 (2007)
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999)
Acknowledgements
We thank E. Bach, D. Godt, S. Hayahsi, N. Perrimon and R. Read for reagents and fly stocks, and Jones laboratory members, G. Adams, M. Buszczak, C. Schulz, S. DiNardo and M. Fuller for discussions and comments on the manuscript. This work was supported by a training grant from the California Institute for Regenerative Medicine to the University of California-San Diego (L. Goldstein). D.L.J. is funded by the Ellison Medical Foundation, the American Federation for Aging Research, the G. Harold and Leila Y. Mathers Charitable Foundation, the ACS and the NIH.
Author Contributions J.V. and D.L.J. planned experiments; J.V. and C.D’A. performed experiments and analysed data; D.L.J. wrote the manuscript; and J.V. and C.D’A. edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information 1
The file contains Supplementary Figures 1-4 and Legends; Supplementary Tables 1-3. The Supplementary Figures include a model of SSC divisions, images of DE-cadherin mutant germline clones, and additional shg RNAi images. The Supplementary Tables include data from clonal analysis, phospho-histone H3, and BrdU experiments. (PDF 9563 kb)
Rights and permissions
About this article
Cite this article
Voog, J., D’Alterio, C. & Jones, D. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature 454, 1132–1136 (2008). https://doi.org/10.1038/nature07173
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07173
This article is cited by
-
A single-cell atlas and lineage analysis of the adult Drosophila ovary
Nature Communications (2020)
-
Ectopic Dpp signaling promotes stem cell competition through EGFR signaling in the Drosophila testis
Scientific Reports (2019)
-
Merlin is required for coordinating proliferation of two stem cell lineages in the Drosophila testis
Scientific Reports (2017)
-
Silver nanoparticles disrupt germline stem cell maintenance in the Drosophila testis
Scientific Reports (2016)
-
The niche in single‐cell technologies
Immunology & Cell Biology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.