Abstract
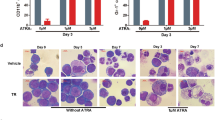
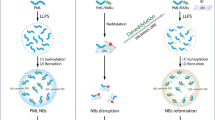
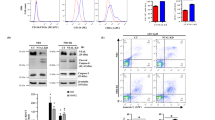
Acute promyelocytic leukemia (APL) is driven by the promyelocytic leukemia (PML)–retinoic acid receptor-α (PML-RARA) fusion protein, which interferes with nuclear receptor signaling and PML nuclear body (NB) assembly. APL is the only malignancy definitively cured by targeted therapies: retinoic acid (RA) and/or arsenic trioxide, which both trigger PML-RARA degradation through nonoverlapping pathways. Yet, the cellular and molecular determinants of treatment efficacy remain disputed. We demonstrate that a functional Pml–transformation-related protein 53 (Trp53) axis is required to eradicate leukemia-initiating cells in a mouse model of APL. Upon RA-induced PML-RARA degradation, normal Pml elicits NB reformation and induces a Trp53 response exhibiting features of senescence but not apoptosis, ultimately abrogating APL-initiating activity. Apart from triggering PML-RARA degradation, arsenic trioxide also targets normal PML to enhance NB reformation, which may explain its clinical potency, alone or with RA. This Pml-Trp53 checkpoint initiated by therapy-triggered NB restoration is specific for PML-RARA–driven APL, but not the RA-resistant promyelocytic leukemia zinc finger (PLZF)-RARA variant. Yet, as NB biogenesis is druggable, it could be therapeutically exploited in non-APL malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
de Thé, H. & Chen, Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat. Rev. Cancer 10, 775–783 (2010).
Licht, J.D. Reconstructing a disease: what essential features of the retinoic acid receptor fusion oncoproteins generate acute promyelocytic leukemia? Cancer Cell 9, 73–74 (2006).
Lallemand-Breitenbach, V. & de The, H. PML nuclear bodies. Cold Spring Harb. Perspect. Biol. 2, a000661 (2010).
Ito, K. et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453, 1072–1078 (2008).
Welch, J.S. et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 150, 264–278 (2012).
Hu, J. et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 106, 3342–3347 (2009).
Lo-Coco, F. et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 369, 111–121 (2013).
Sanz, M.A. et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 113, 1875–1891 (2009).
Zhu, J., Chen, Z., Lallemand-Breitenbach, V. & de Thé, H. How acute promyelocytic leukemia revived arsenic. Nat. Rev. Cancer 2, 705–713 (2002).
Zhu, J. et al. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 94, 3978–3983 (1997).
Jeanne, M. et al. PML-RARA oxidation and arsenic binding initiate the antileukemia response of As2O3 . Cancer Cell 18, 88–98 (2010).
Zhang, X.W. et al. Arsenic trioxide controls the fate of the PML-RARα oncoprotein by directly binding PML. Science 328, 240–243 (2010).
Lallemand-Breitenbach, V. et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML-retinoic acid receptor α degradation. J. Exp. Med. 193, 1361–1371 (2001).
Mathews, V. et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J. Clin. Oncol. 28, 3866–3871 (2010).
Ghavamzadeh, A. et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J. Clin. Oncol. 29, 2753–2757 (2011).
Nasr, R. et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat. Med. 14, 1333–1342 (2008).
Kogan, S.C. Curing APL: differentiation or destruction? Cancer Cell 15, 7–8 (2009).
Ablain, J. et al. Uncoupling RARA transcriptional activation and degradation clarifies the bases for APL response to therapies. J. Exp. Med. 210, 647–653 (2013).
Lallemand-Breitenbach, V. et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J. Exp. Med. 189, 1043–1052 (1999).
Shen, Z.X. et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 101, 5328–5335 (2004).
Estey, E. et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 107, 3469–3473 (2006).
Rego, E.M., He, L.Z., Warrell, R.P. Jr., Wang, Z.G. & Pandolfi, P.P. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARα and PLZF-RARα oncoproteins. Proc. Natl. Acad. Sci. USA 97, 10173–10178 (2000).
Koken, M.H.M. et al. Retinoic acid, but not arsenic trioxide, degrades the PLZF-RARα fusion protein, without inducing terminal differentiation or apoptosis, in a RA-therapy resistant tt(11;17)(q23;q21) APL patient. Oncogene 18, 1113–1118 (1999).
Zhu, J. et al. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor α (RAR α) and oncogenic RAR α fusion proteins. Proc. Natl. Acad. Sci. USA 96, 14807–14812 (1999).
He, L.Z. et al. Two critical hits for promyelocytic leukemia. Mol. Cell 6, 1131–1141 (2000).
Kortlever, R.M., Higgins, P.J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8, 877–884 (2006).
Furze, R.C. & Rankin, S.M. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 22, 3111–3119 (2008).
Freund, A., Laberge, R.M., Demaria, M. & Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066–2075 (2012).
Ivanov, A. et al. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 202, 129–143 (2013).
Minucci, S. et al. PML-RAR induces promyelocytic leukemias with high efficiency following retroviral gene transfer into purified murine hematopoietic progenitors. Blood 100, 2989–2995 (2002).
Bernardi, R. et al. PML regulates p53 stability by sequestering Mdm2 to the nucleolus. Nat. Cell Biol. 6, 665–672 (2004).
Vernier, M. et al. Regulation of E2Fs and senescence by PML nuclear bodies. Genes Dev. 25, 41–50 (2011).
Bischof, O., Nacerddine, K. & Dejean, A. Human papillomavirus oncoprotein E7 targets the promyelocytic leukemia protein and circumvents cellular senescence via the Rb and p53 tumor suppressor pathways. Mol. Cell. Biol. 25, 1013–1024 (2005).
Bischof, O. et al. Deconstructing PML-induced premature senescence. EMBO J. 21, 3358–3369 (2002).
Condemine, W. et al. Characterization of endogenous human promyelocytic leukemia isoforms. Cancer Res. 66, 6192–6198 (2006).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML-RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10, 547–555 (2008).
Stadler, M. et al. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene 11, 2565–2573 (1995).
Zheng, P.Z. et al. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 102, 7653–7658 (2005).
Insinga, A. et al. Impairment of p53 acetylation, stability and function by an oncogenic transcription factor. EMBO J. 23, 1144–1154 (2004).
Zhao, Z. et al. p53 loss promotes acute myeloid leukemia by enabling aberrant self-renewal. Genes Dev. 24, 1389–1402 (2010).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Guidez, F. et al. RARα-PLZF overcomes PLZF-mediated repression of CRABPI, contributing to retinoid resistance in t(11;17) acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 104, 18694–18699 (2007).
Akagi, T. et al. Hidden abnormalities and novel classification of t(15;17) acute promyelocytic leukemia (APL) based on genomic alterations. Blood 113, 1741–1748 (2009).
Gurrieri, C. et al. Mutations of the PML tumor suppressor gene in acute promyelocytic leukemia. Blood 103, 2358–2362 (2004).
Muindi, J. et al. Continuous treatment with all-trans retinoic acid causes a progressive reduction in plasma drug concentrations: implications for relapse and retinoid “resistance” in patients with acute promyelocytic leukemia. Blood 79, 299–303 (1992).
Tsimberidou, A.M. et al. Single-agent liposomal all-trans retinoic acid can cure some patients with untreated acute promyelocytic leukemia: an update of The University of Texas M. D. Anderson Cancer Center Series. Leuk. Lymphoma 47, 1062–1068 (2006).
Stein, E. et al. The coagulopathy of acute promyelocytic leukaemia revisited. Best Pract. Res. Clin. Haematol. 22, 153–163 (2009).
Ferbeyre, G. et al. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14, 2015–2027 (2000).
Pearson, M. et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406, 207–210 (2000).
Guo, A. et al. The function of PML in p53-dependent apoptosis. Nat. Cell Biol. 2, 730–736 (2000).
Nardella, C., Clohessy, J.G., Alimonti, A. & Pandolfi, P.P. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer 11, 503–511 (2011).
Meani, N. et al. Molecular signature of retinoic acid treatment in acute promyelocytic leukemia. Oncogene 24, 3358–3368 (2005).
Sahin, U. et al. Oxidative stress-induced assembly of PML nuclear bodies controls sumoylation of partner proteins. J. Cell Biol (in the press).
El Hajj, H. et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 207, 2785–2792 (2010).
Kchour, G. et al. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon α, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood 113, 6528–6532 (2009).
El Eit, R.M. et al. Effective targeting of chronic myeloid leukemia initiating activity with the combination of arsenic trioxide and interferon α. Int. J. Cancer 134, 988–996 (2014).
Bracken, A.P., Ciro, M., Cocito, A. & Helin, K. E2F target genes: unraveling the biology. Trends Biochem. Sci. 29, 409–417 (2004).
Coppé, J.P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Ragazzon, B. et al. Transcriptome analysis reveals that p53 and β-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Res. 70, 8276–8281 (2010).
Acknowledgements
The laboratory of H.d.T. is supported by the Ligue Nationale contre le Cancer, the Cartes d'Identité des Tumeurs program, the Institut National de la Santé et de la Recherché Médicale (INSERM), the Centre National de la Recherché Scientifique (CNRS), University Paris Diderot, Institut Universitaire de France, Institut National du Cancer, Fondation Association pour la Recherche contre le Cancer (ARC) (Prix Griffuel) and the European Research Council (senior grant 268729 – STEMAPL). J.A. was supported by a fellowship from Ecole Polytechnique and Fondation ARC, K.R. by a fellowship from the Lady Tata and ARC Foundations. S.M. is supported by grants from EPIGEN and the Italian Association for Cancer Research (AIRC). We thank A. Janin, F. Bouhidel and P. Bertheau for assistance with mouse pathology; P. Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire) for RARA-specific antibody; S. Lowe (Memorial Sloan-Kettering Cancer Center) for shRNA vectors and Pml-specific antibody; L. Peres and S. Gressens for technical help; M. Pla for the animal facility; N. Setterblad for imaging; E. Del Neyri and V. Dessirier for imaging statistical analysis; and I. Pallavicini and A. Marinelli for mouse work in Milan. We thank E. Raffoux, C. Bailly, P. Fenaux and N. Boissel (Hôpital St. Louis) for providing the patients' blood samples. We thank R. Ohki and L. Attardi for sharing unpublished p53 ChIP-Seq data. We thank all members of the laboratory of H.d.T. for helpful discussions, J. Godet for continuous support and V. Lallemand-Breitenbach, U. Sahin, S. Benhenda, F. Sigaux and J.C. Gluckman for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
J.A., H.S. and K.R. performed the experiments, J.A., A.d.R. and H.d.T. analyzed the bioinformatic data, S.M. provided reagents and discussed results and J.A., K.R. and H.d.T. analyzed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 1202 kb)
Rights and permissions
About this article
Cite this article
Ablain, J., Rice, K., Soilihi, H. et al. Activation of a promyelocytic leukemia–tumor protein 53 axis underlies acute promyelocytic leukemia cure. Nat Med 20, 167–174 (2014). https://doi.org/10.1038/nm.3441
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3441
This article is cited by
-
Mineral medicine: from traditional drugs to multifunctional delivery systems
Chinese Medicine (2022)
-
Stages of preadipocyte differentiation: biomarkers and pathways for extracellular structural remodeling
Hereditas (2022)
-
Deneddylation of PML/RARα reconstructs functional PML nuclear bodies via orchestrating phase separation to eradicate APL
Cell Death & Differentiation (2022)
-
ACSL1 promotes imatinib-induced chronic myeloid leukemia cell senescence by regulating SIRT1/p53/p21 pathway
Scientific Reports (2022)
-
A novel fusion protein TBLR1-RARα acts as an oncogene to induce murine promyelocytic leukemia: identification and treatment strategies
Cell Death & Disease (2021)