Abstract
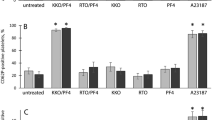
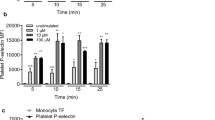
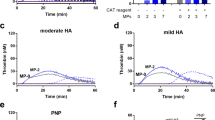
High plasma levels of soluble P-selectin are associated with thrombotic disorders and may predict future cardiovascular events. Mice with high levels of soluble P-selectin have more microparticles in their plasma than do normal mice. Here we show that chimeras of P-selectin and immunoglobulin (P-sel–Ig) induced formation of procoagulant microparticles in human blood through P-selectin glycoprotein ligand-1 (PSGL-1; encoded by the Psgl1 gene, officially known as Selpl). In addition, Psgl1−/− mice produced fewer microparticles after P-sel–Ig infusion and did not spontaneously increase their microparticle count in old age as do wild-type mice. Injected microparticles specifically bound to thrombi and thus could be involved in thrombin generation at sites of injury. Infusion of P-sel–Ig into hemophilia A mice produced a 20-fold increase over control immunoglobulin in microparticles containing tissue factor. This significantly improved the kinetics of fibrin formation in the hemophilia A mice and normalized their tail-bleeding time. P-sel–Ig treatment could become a new approach to sustained control of bleeding in hemophilia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McEver, R.P. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb. Haemost. 86, 746–756 (2001).
Palabrica, T. et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 359, 848–851 (1992).
Mayadas, T.N., Johnson, R.C., Rayburn, H., Hynes, R.O. & Wagner, D.D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 74, 541–554 (1993).
Dunlop, L.C. et al. Characterization of GMP-140 (P-selectin) as a circulating plasma protein. J. Exp. Med. 175, 1147–1150 (1992).
Mehta, P., Patel, K.D., Laue, T.M., Erickson, H.P. & McEver, R.P. Soluble monomeric P-selectin containing only the lectin and epidermal growth factor domains binds to P-selectin glycoprotein ligand-1 on leukocytes. Blood 90, 2381–2389 (1997).
Hartwell, D.W. et al. Role of P-selectin cytoplasmic domain in granular targeting in vivo and in early inflammatory responses. J. Cell Biol. 143, 1129–1141 (1998).
Andre, P., Hartwell, D., Hrachovinova, I., Saffaripour, S. & Wagner, D.D. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc. Natl. Acad. Sci. USA 97, 13835–13840 (2000).
Chong, B.H. et al. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood 83, 1535–1541 (1994).
Toombs, C.F. et al. Pretreatment with a blocking monoclonal antibody to P-selectin accelerates pharmacological thrombolysis in a primate model of arterial thrombosis. J. Pharmacol. Exp. Ther. 275, 941–949 (1995).
Wakefield, T.W. et al. P-selectin and TNF inhibition reduce venous thrombosis inflammation. J. Surg. Res. 64, 26–31 (1996).
Nemerson, Y. Tissue factor and hemostasis. Blood 71, 1–8 (1988).
Hidari, K.I., Weyrich, A.S., Zimmerman, G.A. & McEver, R.P. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J. Biol. Chem. 272, 28750–28756 (1997).
Evangelista, V. et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood 93, 876–885 (1999).
Sako, D. et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell 75, 1179–1186 (1993).
Giesen, P.L. et al. Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. USA 96, 2311–2315 (1999).
Hahne, M., Jager, U., Isenmann, S., Hallmann, R. & Vestweber, D. Five tumor necrosis factor-inducible cell adhesion mechanisms on the surface of mouse endothelioma cells mediate the binding of leukocytes. J. Cell Biol. 121, 655–664 (1993).
Salzman, E.W. & Hirsh, J. The epidemiology, pathogenesis, and natural history of venous thrombosis. in Hemostasis and Thrombosis: Basic principles and clinical practice (eds. Colman, R.W., Hirsh, J., Marder, V.J. & Salzman, E.W.) 1275–1289 (Lippincott, Philadelphia, 1994).
Denis, C. et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc. Natl. Acad. Sci. USA 95, 9524–9529 (1998).
Hafezi-Moghadam, A. & Ley, K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J. Exp. Med. 189, 939–948 (1999).
Bi, L. et al. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat. Genet. 10, 119–121 (1995).
Muchitsch, E.M. et al. Phenotypic expression of murine hemophilia. Thromb. Haemost. 82, 1371–1373 (1999).
Gregory, S.A., Morrissey, J.H. & Edgington, T.S. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol. Cell. Biol. 9, 2752–2755 (1989).
Celi, A. et al. P-selectin induces the expression of tissue factor on monocytes. Proc. Natl. Acad. Sci. USA 91, 8767–8771 (1994).
Piccardoni, P. et al. Platelet/polymorphonuclear leukocyte adhesion: a new role for SRC kinases in Mac-1 adhesive function triggered by P-selectin. Blood 98, 108–116 (2001).
Ushiyama, S., Laue, T.M., Moore, K.L., Erickson, H.P. & McEver, R.P. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J. Biol. Chem. 268, 15229–15237 (1993).
Barkalow, F.J., Barkalow, K.L. & Mayadas, T.N. Dimerization of P-selectin in platelets and endothelial cells. Blood 96, 3070–3077 (2000).
Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 9, 585–590 (1999).
Ishiwata, N. et al. Alternatively spliced isoform of P-selectin is present in vivo as a soluble molecule. J. Biol. Chem. 269, 23708–23715 (1994).
Xia, L. et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J. Clin. Invest. 109, 939–950 (2002).
Ikeda, H. et al. Increased soluble form of P-selectin in patients with unstable angina. Circulation 92, 1693–1696 (1995).
Parker, C., Vita, J.A. & Freedman, J.E. Soluble adhesion molecules and unstable coronary artery disease. Atherosclerosis 156, 417–424 (2001).
Burger, P.C. & Wagner, D.D. Platelet P-selectin facilitates atherosclerotic lesion development. Blood 101, 2661–2666 (2003).
Hillis, G.S. et al. Elevated soluble P-selectin levels are associated with an increased risk of early adverse events in patients with presumed myocardial ischemia. Am. Heart J. 143, 235–241 (2002).
Ridker, P.M., Buring, J.E. & Rifai, N. Soluble P-selectin and the risk of future cardiovascular events. Circulation 103, 491–495 (2001).
Michelson, A.D., Barnard, M.R., Krueger, L.A., Valeri, C.R. & Furman, M.I. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 104, 1533–1537 (2001).
Kumar, A., Villani, M.P., Patel, U.K., Keith, J.C., Jr. & Schaub, R.G. Recombinant soluble form of PSGL-1 accelerates thrombolysis and prevents reocclusion in a porcine model. Circulation 99, 1363–1369 (1999).
McEver, R.P. P-selectin and PSGL-1: exploiting connections between inflammation and venous thrombosis. Thromb. Haemost. 87, 364–365 (2002).
Myers, D. et al. New and effective treatment of experimentally induced venous thrombosis with anti-inflammatory rPSGL-Ig. Thromb. Haemost. 87, 374–382 (2002).
Falati, S., Gross, P., Merrill-Skoloff, G., Furie, B.C. & Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8, 1175–1181 (2002).
Rauch, U. et al. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood 96, 170–175 (2000).
Kung, S.H. et al. Human factor IX corrects the bleeding diathesis of mice with hemophilia B. Blood 91, 784–790 (1998).
Mannucci, P.M. & Tuddenham, E.G. The hemophilias—from royal genes to gene therapy. N. Engl. J. Med. 344, 1773–1779 (2001).
Lacroix-Desmazes, S. et al. Pathophysiology of inhibitors to factor VIII in patients with haemophilia A. Haemophilia 8, 273–279 (2002).
DiMichele, D.M. Inhibitors in haemophilia: a primer. Haemophilia 6 (suppl. 1), 38–40 (2000).
Prorock, A.J., Hafezi-Moghadam, A., Laubach, V.E., Liao, J.K. & Ley, K. Vascular protection by estrogen in ischemia-reperfusion injury requires endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 284, H133–H140 (2003).
Acknowledgements
We thank W. Ruf for advice and control reagents for tissue factor studies, P. Salaj and Z. Vorlová for procuring blood samples from hemophilia A patients, A. Kumar for helpful discussions, S. Saffaripour and K. Thomas for technical assistance, and L. Cowan and T. Takagi for help preparing the manuscript. This work was supported in part by National Institutes of Health, National Heart, Lung and Blood Institute grants HL 54502 (R.P.M.) and HL56949 and 53756 (D.D.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.G.S. is an employee of Wyeth Research. R.T.C. and A.W. were employees of Wyeth Research while the study was performed.
Rights and permissions
About this article
Cite this article
Hrachovinová, I., Cambien, B., Hafezi-Moghadam, A. et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med 9, 1020–1025 (2003). https://doi.org/10.1038/nm899
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm899
This article is cited by
-
The roles of P-selectin in cancer cachexia
Medical Oncology (2023)
-
Phosphatidylserine Regulation of Coagulation Proteins Factor IXa and Factor VIIIa
The Journal of Membrane Biology (2022)
-
Phosphatidylserine positive microparticles improve hemostasis in in-vitro hemophilia A plasma models
Scientific Reports (2020)
-
LPS-induced expression and release of monocyte tissue factor in patients with haemophilia
Annals of Hematology (2020)
-
Extracellular vesicles: how they interact with endothelium, potentially contributing to metastatic cancer cell implants
Clinical and Translational Medicine (2017)