Abstract
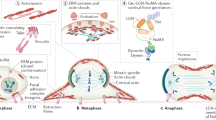
The spatio-temporal regulation of symmetrical as opposed to asymmetric cell divisions directs the fate and location of cells in the developing CNS. In invertebrates, G-protein regulators control spindle orientation in asymmetric divisions, which generate progeny with different identities. We investigated the role of the G-protein regulator LGN (also called Gpsm2) in spindle orientation and cell-fate determination in the spinal cord neuroepithelium of the developing chick embryo. We show that LGN is located at the cell cortex and spindle poles of neural progenitors, and that it regulates spindle movements and orientation. LGN promotes planar divisions in the early spinal cord. Interfering with LGN function randomizes the plane of division. Notably, this does not affect cell fate, but frequently leads one daughter of proliferative symmetric divisions to exit the neuroepithelium prematurely and to proliferate aberrantly in the mantle zone. Hence, tight control of planar spindle orientation maintains neural progenitors in the neuroepithelium, and regulates the proper development of the nervous system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988).
McConnell, S.K. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron 15, 761–768 (1995).
Fishell, G. & Kriegstein, A.R. Neurons from radial glia: the consequences of asymmetric inheritance. Curr. Opin. Neurobiol. 13, 34–41 (2003).
Huttner, W.B. & Brand, M. Asymmetric division and polarity of neuroepithelial cells. Curr. Opin. Neurobiol. 7, 29–39 (1997).
Gotz, M. & Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788 (2005).
Chenn, A. & McConnell, S.K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 82, 631–641 (1995).
Kosodo, Y. et al. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 23, 2314–2324 (2004).
Huttner, W.B. & Kosodo, Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 17, 648–657 (2005).
Fish, J.L., Kosodo, Y., Enard, W., Paabo, S. & Huttner, W.B. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA 103, 10438–10443 (2006).
Sanada, K. & Tsai, L.H. G protein βχ subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122, 119–131 (2005).
Bellaiche, Y. & Gotta, M. Heterotrimeric G proteins and regulation of size asymmetry during cell division. Curr. Opin. Cell Biol. 17, 658–663 (2005).
Yu, F., Kuo, C.T. & Jan, Y.N. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron 51, 13–20 (2006).
Schaefer, M., Petronczki, M., Dorner, D., Forte, M. & Knoblich, J.A. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell 107, 183–194 (2001).
Fuse, N., Hisata, K., Katzen, A.L. & Matsuzaki, F. Heterotrimeric G proteins regulate daughter cell size asymmetry in Drosophila neuroblast divisions. Curr. Biol. 13, 947–954 (2003).
Izumi, Y., Ohta, N., Itoh-Furuya, A., Fuse, N. & Matsuzaki, F. Differential functions of G protein and Baz-aPKC signaling pathways in Drosophila neuroblast asymmetric division. J. Cell Biol. 164, 729–738 (2004).
Schaefer, M., Shevchenko, A. & Knoblich, J.A. A protein complex containing Inscuteable and the Gα-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr. Biol. 10, 353–362 (2000).
Yu, F., Morin, X., Cai, Y., Yang, X. & Chia, W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell 100, 399–409 (2000).
Yu, F. et al. Locomotion defects, together with Pins, regulates heterotrimeric G-protein signaling during Drosophila neuroblast asymmetric divisions. Genes Dev. 19, 1341–1353 (2005).
Wang, H. et al. Ric-8 controls Drosophila neural progenitor asymmetric division by regulating heterotrimeric G proteins. Nat. Cell Biol. 7, 1091–1098 (2005).
Hampoelz, B., Hoeller, O., Bowman, S.K., Dunican, D. & Knoblich, J.A. Drosophila Ric-8 is essential for plasma-membrane localization of heterotrimeric G proteins. Nat. Cell Biol. 7, 1099–1105 (2005).
Bowman, S.K., Neumuller, R.A., Novatchkova, M., Du, Q. & Knoblich, J.A. The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell 10, 731–742 (2006).
Izumi, Y., Ohta, N., Hisata, K., Raabe, T. & Matsuzaki, F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat. Cell Biol. 8, 586–593 (2006).
Siller, K.H., Cabernard, C. & Doe, C.Q. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat. Cell Biol. 8, 594–600 (2006).
Colombo, K. et al. Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science 300, 1957–1961 (2003).
Gotta, M., Dong, Y., Peterson, Y.K., Lanier, S.M. & Ahringer, J. Asymmetrically distributed C. elegans homologs of AGS3/PINS control spindle position in the early embryo. Curr. Biol. 13, 1029–1037 (2003).
Voronina, E. & Wessel, G.M. Activator of G protein signaling in asymmetric cell divisions of the sea urchin embryo. Dev. Growth Differ. 48, 549–557 (2006).
Mochizuki, N., Cho, G., Wen, B. & Insel, P.A. Identification and cDNA cloning of a novel human mosaic protein, LGN, based on interaction with G alpha i2. Gene 181, 39–43 (1996).
Takesono, A. et al. Receptor-independent activators of heterotrimeric G protein signaling pathways. J. Biol. Chem. 274, 33202–33205 (1999).
Du, Q., Stukenberg, P.T. & Macara, I.G. A mammalian partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat. Cell Biol. 3, 1069–1075 (2001).
Siderovski, D.P., Diverse-Pierluissi, M. & De Vries, L. The GoLoco motif: a Galphai/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem. Sci. 24, 340–341 (1999).
Willard, F.S., Kimple, R.J. & Siderovski, D.P. Return of the GDI: the GoLoco motif in cell division. Annu. Rev. Biochem. 73, 925–951 (2004).
Blumer, J.B., Kuriyama, R., Gettys, T.W. & Lanier, S.M. The G protein regulatory (GPR) motif-containing Leu-Gly-Asn-enriched protein (LGN) and Giα3 influence cortical positioning of the mitotic spindle poles at metaphase in symmetrically dividing mammalian cells. Eur. J. Cell Biol. 85, 1233–1240 (2006).
Du, Q. & Macara, I.G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516 (2004).
Kaushik, R., Yu, F., Chia, W., Yang, X. & Bahri, S. Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression. Mol. Biol. Cell 14, 3144–3155 (2003).
Yu, F. et al. A mouse homologue of Drosophila pins can asymmetrically localize and substitute for pins function in Drosophila neuroblasts. J. Cell Sci. 116, 887–896 (2003).
Blumer, J.B., Chandler, L.J. & Lanier, S.M. Expression analysis and subcellular distribution of the two G protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J. Biol. Chem. 277, 15897–15903 (2002).
Das, R.M. et al. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev. Biol. 294, 554–563 (2006).
Reinsch, S. & Karsenti, E. Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J. Cell Biol. 126, 1509–1526 (1994).
Adams, R.J. Metaphase spindles rotate in the neuroepithelium of rat cerebral cortex. J. Neurosci. 16, 7610–7618 (1996).
Haydar, T.F., Ang, E., Jr. & Rakic, P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc. Natl. Acad. Sci. USA 100, 2890–2895 (2003).
Roszko, I., Afonso, C., Henrique, D. & Mathis, L. Key role played by RhoA in the balance between planar and apico-basal cell divisions in the chick neuroepithelium. Dev. Biol. 298, 212–224 (2006).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Kraut, R., Chia, W., Jan, L.Y., Jan, Y.N. & Knoblich, J.A. Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature 383, 50–55 (1996).
Zigman, M. et al. Mammalian inscuteable regulates spindle orientation and cell fate in the developing retina. Neuron 48, 539–545 (2005).
Yu, F., Ong, C.T., Chia, W. & Yang, X. Membrane targeting and asymmetric localization of Drosophila partner of inscuteable are discrete steps controlled by distinct regions of the protein. Mol. Cell. Biol. 22, 4230–4240 (2002).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Pizzinat, N., Takesono, A. & Lanier, S.M. Identification of a truncated form of the G protein regulator AGS3 in heart that lacks the tetratricopeptide repeat domains. J. Biol. Chem. 276, 16601–16610 (2001).
Cai, Y., Yu, F., Lin, S., Chia, W. & Yang, X. Apical complex genes control mitotic spindle geometry and relative size of daughter cells in Drosophila neuroblast and pI asymmetric divisions. Cell 112, 51–62 (2003).
Wilcock, A.C., Swedlow, J.R. & Storey, K.G. Mitotic spindle orientation distinguishes stem cell and terminal modes of neuron production in the early spinal cord. Development 134, 1943–1954 (2007).
Hilgers, V., Pourquie, O. & Dubrulle, J. In vivo analysis of mRNA stability using the Tet-Off system in the chicken embryo. Dev. Biol. 284, 292–300 (2005).
Acknowledgements
This work was supported by the French Centre National de la Recherche Scientifique and an Association Française contre les Myopathies grant (11942-SR-C) to X.M. F.J. is the recipient of a PhD fellowship from the French Ministry for Higher Education and Research. We thank J. Hazan (King's College London, UK) for RT-PCR cloning of the full-length chick LGN cDNA, D. Henrique (University of Lisbon, Portugal) for the hes5-1 plasmid, K. Hadjantonakis (Sloan-Kettering Institute, New York) for the pCX-H2B-EGFP plasmid, F. Yu (Temasek Life Sciences Laboratory, Singapore) and R. Kaushik (IMCB, Singapore) for mouse LGN cDNA, and the PICsL imaging core facility for expert technical assistance. We thank C. Goridis and V. Dubreuil (Ecole Normale Supérieure, Paris), W. Chia (Temasek Life Sciences Laboratory, Singapore), A. Moqrich (IBDML Marseille) and members of the Durbec group for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
X.M. and P.D. supervised the project, X.M. and F.J. performed the experiments and X.M. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 456 kb)
Supplementary Video 1
Time-lapse analysis of metaphase movements in a control embryo. H2B-EGFP is used as a chromosomal reporter. The ventricular surface (apical) is on top. Each frame is a projection of a 21-μm-thick stack of confocal sections taken at 1-μm z-intervals. Frames were taken at 1-min intervals over a 200-min period. Most metaphase nuclei display strong rotation and oscillatory movements. Colored asterisks mark several representative cells during prophase, and are erased at the onset of metaphase for better visualization of metaphase movements and anaphase. (MOV 3007 kb)
Supplementary Video 2
Time-lapse analysis of metaphase movements in a Ct-cLGN expressing embryo. H2B-EGFP is used as a chromosomal reporter. The ventricular surface (apical) is on top. Each frame is a projection of a 21-μm-thick stack of confocal sections taken at 1-μm z intervals. Frames were taken at 1-min intervals over a 180-min period. Metaphase nuclei display very weak oscillatory movements. Colored asterisks mark several representative cells during prophase, and are erased at the onset of metaphase for better visualization of metaphase movements and anaphase. (MOV 2787 kb)
Rights and permissions
About this article
Cite this article
Morin, X., Jaouen, F. & Durbec, P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci 10, 1440–1448 (2007). https://doi.org/10.1038/nn1984
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1984
This article is cited by
-
Identification of hub genes associated with prognosis, diagnosis, immune infiltration and therapeutic drug in liver cancer by integrated analysis
Human Genomics (2021)
-
Spindle positioning and its impact on vertebrate tissue architecture and cell fate
Nature Reviews Molecular Cell Biology (2021)
-
Endfoot regeneration restricts radial glial state and prevents translocation into the outer subventricular zone in early mammalian brain development
Nature Cell Biology (2020)
-
Evolutionary modification of AGS protein contributes to formation of micromeres in sea urchins
Nature Communications (2019)
-
Live imaging screen reveals that TYRO3 and GAK ensure accurate spindle positioning in human cells
Nature Communications (2019)