Key Points
-
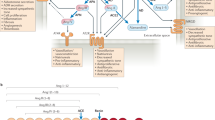
Junctional adhesion molecules (JAMs) are expressed by leukocytes, platelets, endothelial and epithelial cells. JAMs are important in the control of vascular permeability and leukocyte transmigration across endothelial-cell surfaces.
-
JAMs engage in homophilic interactions and various heterophilic interactions with the leukocyte integrins lymphocyte function-associated antigen 1 (LFA1), very late antigen 4 (VLA4) and MAC1.
-
JAMs are upregulated under inflammatory, atherosclerotic or ischaemic conditions. JAMs are redistributed from intercellular junctions to the cell surface after inflammatory stimulation.
-
Both endothelial-cell and leukocyte JAM-A contribute in a molecular 'zipper' that controls transendothelial diapedesis, whereas JAM-C supports leukocyte adhesion on platelets and subsequent transendothelial migration. Both JAM-A and JAM-C promote leukocyte recruitment on activated endothelium under inflammatory conditions.
-
Studies using genetically modified mice have shown important roles for JAMs in the regulation of leukocyte recruitment to sites of inflammation, ischaemia–reperfusion injury, atherogenesis, and in neointima formation.
-
JAMs are involved in growth-factor-mediated angiogenesis by association with αvβ3-integrin and subsequent signalling steps.
Abstract
Junctional adhesion molecules (JAMs) of the immunoglobulin superfamily are important in the control of vascular permeability and leukocyte transmigration across endothelial-cell surfaces, by engaging in homophilic, heterophilic and lateral interactions. Through their localization on the endothelial-cell surface and expression by platelets, JAMs contribute to adhesive interactions with circulating leukocytes and platelets. Antibody-blocking studies and studies using genetically modified mice have implicated these functions of JAMs in the regulation of leukocyte recruitment to sites of inflammation and ischaemia–reperfusion injury, in growth-factor-mediated angiogenesis, atherogenesis and neointima formation. The comparison of different JAM-family members and animal models, however, shows that the picture remains rather complex. This Review summarizes recent progress and future directions in understanding the role of JAMs as 'gate keepers' in inflammation and vascular pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Muller, W. A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24, 327–334 (2003).
Imhof, B. A. & Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nature Rev. Immunol. 4, 432–444 (2004).
Weber, C. Novel mechanistic concepts for the control of leukocyte transmigration: specialization of integrins, chemokines, and junctional molecules. J. Mol. Med. 81, 4–19 (2003).
Bazzoni, G. The JAM family of junctional adhesion molecules. Curr. Opin. Cell Biol. 15, 525–530 (2003).
Martin-Padura, I. et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142, 117–127 (1998). This paper reports the identification of JAM-A at intercellular junctions and its involvement in mononuclear transmigration.
Williams, L. A., Martin-Padura, I., Dejana, E., Hogg, N. & Simmons, D. L. Identification and characterisation of human junctional adhesion molecule (JAM). Mol. Immunol. 36, 1175–1188 (1999).
Palmeri, D., van Zante, A., Huang, C. C., Hemmerich, S. & Rosen, S. D. Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J. Biol. Chem. 275, 19139–19145 (2000).
Cunningham, S. A. et al. A novel protein with homology to the junctional adhesion molecule. Characterization of leukocyte interactions. J. Biol. Chem. 275, 34750–34756 (2000).
Aurrand-Lions, M., Duncan, L., Ballestrem, C. & Imhof, B. A. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem. 276, 2733–2741 (2001).
Arrate, M. P., Rodriguez, J. M., Tran, T. M., Brock, T. A. & Cunningham, S. A. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J. Biol. Chem. 276, 45826–45832 (2001).
Liang, T. W. et al. Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM3. J. Immunol. 168, 1618–1626 (2002).
Hirabayashi, S. et al. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol. Cell. Biol. 23, 4267–4282 (2003).
Moog-Lutz, C. et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood 102, 3371–3378 (2003).
Kostrewa, D. et al. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. Embo J. 20, 4391–4398 (2001).
Prota, A. E. et al. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc. Natl Acad. Sci. USA 100, 5366–5371 (2003).
Liu, Y. et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113 2363–2374 (2000).
Johnson-Leger, C. A., Aurrand-Lions, M., Beltraminelli, N., Fasel, N. & Imhof, B. A. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood 100, 2479–2486 (2002).
Santoso, S. et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 196, 679–691 (2002). In this paper the leukocyte integrin MAC1 is reported to act as a receptor for JAM-C, which supports a crucial role for JAMs in platelet-mediated leukocyte recruitment.
Aurrand-Lions, M. et al. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J. Immunol. 174, 6406–6415 (2005).
Bazzoni, G. et al. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 275, 20520–20526 (2000).
Ebnet, K., Schulz, C. U., Meyer Zu Brickwedde, M. K., Pendl, G. G. & Vestweber, D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275, 27979–27988 (2000).
Ebnet, K. et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). Embo J. 20, 3738–3748 (2001).
Zen, K. et al. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol. Biol. Cell 15, 3926–3937 (2004).
Ebnet, K. et al. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J. Cell Sci. 116, 3879–3891 (2003).
Mandicourt, G., Iden, S., Ebnet, K., Aurrand-Lions, M. & Imhof, B. A. JAM-C regulates tight junctions and integrin-mediated cell adhesion and migration. J. Biol. Chem. 282, 1830–1837 (2006).
Ozaki, H. et al. Cutting edge: combined treatment of TNF-α and IFN-γ causes redistribution of junctional adhesion molecule in human endothelial cells. J. Immunol. 163, 553–557 (1999).
Ostermann, G., Weber, K. S., Zernecke, A., Schroder, A. & Weber, C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nature Immunol. 3, 151–158 (2002). This paper identifies JAM-A as a new ligand for the leukocyte integrin LFA1 and provides the first evidence for a functional relevance of heterophilic JAM interactions.
Shaw, S. K. et al. Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-α plus interferon-γ does not reduce leukocyte transmigration under flow. Am. J. Pathol. 159, 2281–2291 (2001).
Naik, M. U., Mousa, S. A., Parkos, C. A. & Naik, U. P. Signaling through JAM-1 and αvβ3 is required for the angiogenic action of bFGF: dissociation of the JAM-1 and αvβ3 complex. Blood 102, 2108–2114 (2003). This paper provides an important description of the JAM-A–α v β 3 -integrin complex and its role in angiogenesis.
Keiper, T. et al. The role of junctional adhesion molecule-C (JAM-C) in oxidized LDL-mediated leukocyte recruitment. Faseb J. 19, 2078–2080 (2005).
Ostermann, G. et al. Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: inhibition by soluble JAM-A. Arterioscler. Thromb. Vasc. Biol. 25, 729–735 (2005).
Slevin, M. et al. Identification of differential protein expression associated with development of unstable human carotid plaques. Am. J. Pathol. 168, 1004–1021 (2006).
Babinska, A. et al. The F11 receptor (F11R/JAM-A) in atherothrombosis: overexpression of F11R in atherosclerotic plaques. Thromb. Haemost. 97, 272–281 (2007).
Khandoga, A. et al. Junctional adhesion molecule-A deficiency increases hepatic ischemia–reperfusion injury despite reduction of neutrophil transendothelial migration. Blood 106, 725–733 (2005). This paper implicates endothelial-cell JAM-A in the extravasation of neutrophils in vivo using genetically deficient mice in a model of ischaemia–reperfusion injury of the liver.
Aurrand-Lions, M., Johnson-Leger, C., Wong, C., Du Pasquier, L. & Imhof, B. A. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood 98, 3699–3707 (2001).
Naik, U. P., Naik, M. U., Eckfeld, K., Martin-DeLeon, P. & Spychala, J. Characterization and chromosomal localization of JAM-1, a platelet receptor for a stimulatory monoclonal antibody. J. Cell Sci. 114, 539–547 (2001).
Bazzoni, G. et al. Homophilic interaction of junctional adhesion molecule. J. Biol. Chem. 275, 30970–30976 (2000).
Babinska, A. et al. F11-receptor (F11R/JAM) mediates platelet adhesion to endothelial cells: role in inflammatory thrombosis. Thromb. Haemost. 88, 843–850 (2002).
Santoso, S. et al. The homophilic binding of junctional adhesion molecule-C mediates tumor cell-endothelial cell interactions. J. Biol. Chem. 280, 36326–36333 (2005).
Lamagna, C. et al. Dual interaction of JAM-C with JAM-B and αMβ2 integrin: function in junctional complexes and leukocyte adhesion. Mol. Biol. Cell 16, 4992–5003 (2005). This paper provides an interesting analysis of the heterophilic interactions of endothelial-cell JAM-B and JAM-C and its modulation of JAM-C interactions with MAC1.
Bazzoni, G. Endothelial tight junctions: permeable barriers of the vessel wall. Thromb. Haemost. 95, 36–42 (2006).
Mandell, K. J., Babbin, B. A., Nusrat, A. & Parkos, C. A. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on β1 integrins and Rap1 activity. J. Biol. Chem. 280, 11665–11674 (2005).
Mandell, K. J., McCall, I. C. & Parkos, C. A. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J. Biol. Chem. 279, 16254–16262 (2004).
Orlova, V. V., Economopoulou, M., Lupu, F., Santoso, S. & Chavakis, T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J. Exp. Med. 203, 2703–2714 (2006).
Del Maschio, A. et al. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM). J. Exp. Med. 190, 1351–1356 (1999).
Lechner, F. et al. Antibodies to the junctional adhesion molecule cause disruption of endothelial cells and do not prevent leukocyte influx into the meninges after viral or bacterial infection. J. Infect. Dis. 182, 978–982 (2000).
Zernecke, A. et al. Importance of junctional adhesion molecule-A for neointimal lesion formation and infiltration in atherosclerosis-prone mice. Arterioscler. Thromb. Vasc. Biol. 26, e10–e13 (2006). This paper reports the first evidence for an important role for JAM-A in neointimal lesion formation and monocyte infiltration after arterial denudation injury.
Chavakis, T. et al. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J. Biol. Chem. 279, 55602–55608 (2004).
Fraemohs, L., Koenen, R. R., Ostermann, G., Heinemann, B. & Weber, C. The functional interaction of the β2 integrin lymphocyte function-associated antigen-1 with junctional adhesion molecule-A is mediated by the I domain. J. Immunol. 173, 6259–6264 (2004).
Naik, M. U. & Naik, U. P. Junctional adhesion molecule-A-induced endothelial cell migration on vitronectin is integrin αvβ3 specific. J. Cell. Sci. 119, 490–499 (2006).
Boettner, B., Govek, E. E., Cross, J. & Van Aelst, L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl Acad. Sci. USA 97, 9064–9069 (2000).
Cunningham, S. A., Rodriguez, J. M., Arrate, M. P., Tran, T. M. & Brock, T. A. JAM2 interacts with α4β1. Facilitation by JAM3. J. Biol. Chem. 277, 27589–27592 (2002).
Shaw, S. K. et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J. Exp. Med. 200, 1571–1580 (2004).
Weber, C., Lu, C. F., Casasnovas, J. M. & Springer, T. A. Role of αLβ2 integrin avidity in transendothelial chemotaxis of mononuclear cells. J. Immunol. 159, 3968–3975 (1997).
Springer, T. A. & Wang, J. H. The three-dimensional structure of integrins and their ligands, and conformational regulation of cell adhesion. Adv. Protein Chem. 68, 29–63 (2004).
Smith, A. et al. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J. Cell Biol. 170, 141–151 (2005).
Wojcikiewicz, E. P., Azad, H., Koenen, R. R., Moy, V. T. & Weber, C. Homophilic and heterophilic interactions of JAM-A measured using atomic force microscopy. Miami Winter Symp. Short Rep. 18, 31 (2007).
Corada, M. et al. Junctional adhesion molecule-A-deficient polymorphonuclear cells show reduced diapedesis in peritonitis and heart ischemia–reperfusion injury. Proc. Natl Acad. Sci. USA 102, 10634–10639 (2005). This paper describes an interesting role of leukocyte JAM-A in neutrophil polarization and directed migration during inflammatory extravasation.
Chavakis, T., Preissner, K. T. & Santoso, S. Leukocyte trans-endothelial migration: JAMs add new pieces to the puzzle. Thromb. Haemost. 89, 13–17 (2003).
von Hundelshausen, P. & Weber, C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ. Res. 100, 27–40 (2007).
Kornecki, E., Walkowiak, B., Naik, U. P. & Ehrlich, Y. H. Activation of human platelets by a stimulatory monoclonal antibody. J. Biol. Chem. 265, 10042–10048 (1990).
Mause, S. F., von Hundelshausen, P., Zernecke, A., Koenen, R. R. & Weber, C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 25, 1512–1518 (2005).
Weber, C. & Springer, T. A. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to αIIbβ3 and stimulated by platelet-activating factor. J. Clin. Invest. 100, 2085–2093 (1997).
Cera, M. R. et al. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J. Clin. Invest. 114, 729–738 (2004).
Cooke, V. G., Naik, M. U. & Naik, U. P. Fibroblast growth factor-2 failed to induce angiogenesis in junctional adhesion molecule-deficient mice. Arterioscler. Thromb. Vasc. Biol. 26, 2005–2011 (2006).
Naik, M. U., Vuppalanchi, D. & Naik, U. P. Essential role of junctional adhesion molecule-1 in basic fibroblast growth factor-induced endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 23, 2165–2171 (2003).
Lamagna, C., Hodivala-Dilke, K. M., Imhof, B. A. & Aurrand-Lions, M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 65, 5703–5710 (2005).
Fuse, C., Ishida, Y., Hikita, T., Asai, T. & Oku, N. Junctional adhesion molecule-C promotes metastatic potential of HT1080 human fibrosarcoma. J. Biol. Chem. 282, 8276–8283 (2007).
Martinez-Mier, G., Toledo-Pereyra, L. H. & Ward, P. A. Adhesion molecules in liver ischemia and reperfusion. J. Surg. Res. 94, 185–194 (2000).
Weber, C., Schober, A. & Zernecke, A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler. Thromb. Vasc. Biol. 24, 1997–2008 (2004).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Pajukanta, P. et al. Familial combined hyperlipidemia is associated with upstream transcription factor 1 (USF1). Nature Genet. 36, 371–376 (2004).
Huertas-Vazquez, A. et al. Familial combined hyperlipidemia in Mexicans: association with upstream transcription factor 1 and linkage on chromosome 16q24.1. Arterioscler. Thromb. Vasc. Biol. 25, 1985–1991 (2005).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nature Med. 9, 61–67 (2003).
von Hundelshausen, P. et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 103, 1772–1777 (2001).
Nasdala, I. et al. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J. Biol. Chem. 277, 16294–16303 (2002).
Wegmann, F. et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J. Exp. Med. 203, 1671–1677 (2006).
Cohen, C. J. et al. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl Acad. Sci. USA 98, 15191–15196 (2001).
Vincent, T., Pettersson, R. F., Crystal, R. G. & Leopold, P. L. Cytokine-mediated downregulation of coxsackievirus-adenovirus receptor in endothelial cells. J. Virol. 78, 8047–8058 (2004).
Coyne, C. B., Voelker, T., Pichla, S. L. & Bergelson, J. M. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP1) within the tight junction. J. Biol. Chem. 279, 48079–48084 (2004).
Mirza, M. et al. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp. Cell Res. 312, 817–830 (2006).
Acknowledgements
The authors thank R. R. Koenen for helpful discussions and E. A. Liehn for help with immunofluorescence.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Adherens junctions
-
Specialized intercellular junctions of the plasma membrane, in which the cadherin molecules at the surface of adjacent cells interact in a calcium-dependent manner. Actin filaments are linked to these cadherin structures through catenin molecules that are located beneath the junctions.
- Tight junctions
-
A belt-like region of adhesion between adjacent epithelial or endothelial cells that regulates paracellular flux. Tight-junction proteins include the integral membrane proteins occludin and claudin, in association with cytoplasmic zonula occludins proteins.
- High endothelial venule
-
A specialized venule that occurs in secondary lymphoid organs, except the spleen. HEVs allow continuous transmigration of lymphocytes as a consequence of the constitutive expression of adhesion molecules and chemokines at their luminal surface.
- Desmosome
-
An adhesive junction that connects epithelial cells in stratified squamous tissues. These junctions are composed of multiple protein subunits and are the points where keratin filaments are inserted into the plasma membrane.
- Haptotactic gradient
-
A substrate-bound gradient that promotes directed migration of adherent cells.
- Atherosclerotic plaque
-
An atherosclerotic lesion consisting of a fibrotic cap surrounding a lipid-rich core. The lesion is the site of inflammation, lipid accumulation and cell death. It is also known as an atheroma.
- Ischaemia–reperfusion injury
-
An injury in which the tissue first suffers from hypoxia as a result of severely decreased, or completely arrested, blood flow. Restoration of normal blood flow then triggers inflammation, which exacerbates the tissue damage.
- Surface plasmon resonance
-
The detection of alterations in plasmon waves generated at a metal–liquid interface. Changes in surface plasmon resonance are a function of the mass of molecules bound to the interface, so this technique allows sensitive detection of ligand binding in real time without requiring the chemical modification of ligands to enable their detection.
- Vascular endothelial cadherin
-
An endothelial-cell-specific cadherin (adhesive protein) that is present in adherens junctions, which are located between endothelial cells.
- Pseudopod
-
A temporary projection of the cytoplasm of certain cells, such as neutrophils, or of certain unicellular organisms, especially amoebae, that functions in locomotion.
- Uropod
-
A slender protrusion that forms at the rear end of migrating leukocytes.
- Neointima
-
The innermost layer of an artery, which is covered by a monolayer of endothelium, is termed the intima. Atherosclerotic plaques form in the intima. A neointima forms after arterial denudation injury comprising a new layer of endothelial cells on the intimal surface.
- Atherothrombosis
-
Following the rupture of unstable atherosclerotic plaques, thrombogenic material becomes exposed or released to mediate thrombus formation and eventually occlusion of an artery.
Rights and permissions
About this article
Cite this article
Weber, C., Fraemohs, L. & Dejana, E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol 7, 467–477 (2007). https://doi.org/10.1038/nri2096
Issue Date:
DOI: https://doi.org/10.1038/nri2096
This article is cited by
-
Embedded macrophages induce intravascular coagulation in 3D blood vessel-on-chip
Biomedical Microdevices (2024)
-
miR-27a inhibits molecular adhesion between monocytes and human umbilical vein endothelial cells; systemic approach
BMC Research Notes (2022)
-
Design of therapeutic biomaterials to control inflammation
Nature Reviews Materials (2022)
-
Tight Junctions, the Epithelial Barrier, and Toll-like Receptor-4 During Lung Injury
Inflammation (2022)
-
Migraine and neuroinflammation: the inflammasome perspective
The Journal of Headache and Pain (2021)