-
PDF
- Split View
-
Views
-
Cite
Cite
Walter F. Schlech, David Acheson, Foodborne Listeriosis, Clinical Infectious Diseases, Volume 31, Issue 3, September 2000, Pages 770–775, https://doi.org/10.1086/314008
Close - Share Icon Share
Abstract
Listeria monocytogenes emerged as an important foodborne pathogen in the latter part of the 20th century. Clinical syndromes caused by this microorganism include sepsis in the immunocompromised patient, meningoencephalitis in infants and adults, and febrile gastroenteritis. Focal infections at other sites are less frequent. Listeria species are commonly found in raw and unprocessed food products. Major outbreaks of listeriosis, with high morbidity and mortality, have been caused by a variety of foods, including soft cheeses, delicatessen meats, and vegetable products. Improved detection methods, dietary recommendations, and, in some cases, preemptive antibiotic treatment or prophylaxis have reduced the incidence of sporadic listeriosis infections in the United States. Microbial virulence factors distinguishing environmental strains of L. monocytogenes from invasive strains causing foodborne illness and host factors promoting human infection remain incompletely understood.
Listeria monocytogenes, an aerobic, gram-positive coccobacillus, has emerged in the last 20 years from relative microbial obscurity to become an important foodborne pathogen of humans. Most foodborne pathogens plaguing human populations cause significant morbidity but little mortality. Listeriosis, however, is a commonly fatal infection of the bloodstream and CNS. Its recent importance has little to do with altered pathogenicity of the organism but everything to do with late 20th century changes in food processing and distribution in the “global village” as well as the increased prevalence of host factors that enhance the risk of infection.
Despite this increased recognition, listeriosis is still an uncommon infection and may not immediately come to mind in the evaluation of the patient with sepsis, meningitis, encephalitis, or febrile gastroenteritis. In particular, the diagnosis of listeriosis may not be considered in the empirical treatment of bacterial meningitis before the organism is identified in blood or CSF, which can lead to delays in diagnosis and inappropriate treatment with a poor outcome. Listeriosis can therefore become a tort lawyer's dream come true, since it raises both the issue of possible product liability of food manufacturers and the issue of possible medical malpractice because of delays in diagnosis and treatment.
Historical Aspects of Listeriosis
L. monocytogenes is a latecomer to the field of bacteriology. The organism was initially described as a cause of epizootics in veld rodents from South Africa (Tiger River disease) by Pirie [1]. In 1926, Murray et al. [2] described a septic illness in laboratory rabbits that was characterized by peripheral monocytosis. For this reason, the organism was called Bacterium monocytogenes until the genus name was changed first to Listerella then to Listeria. In human infection, monocytosis is not a defining part of the clinical syndrome called listeriosis, although a monocytosis-producing antigen has been characterized from the organism [3].
Some clinical descriptions of both animal and human disease caused by L. monocytogenes were published in the 1920s; however, the organism remained a laboratory curiosity until the World War II era, when it was described as an important cause of neonatal sepsis and meningitis in postwar East Germany [4]. With the development of powerful immunosuppressive drugs, such as corticosteroids, and chemotherapy for malignancy in the 1950s and 1960s, listeriosis in adult patients with compromised immune systems was finally recognized [5]. The development of renal dialysis, and, later, solid organ transplantation, expanded the host population at risk for listeriosis [6]. In the last 20 years, the HIV epidemic has added an additional population at risk, whose excess morbidity due to listeriosis is 500-fold greater than that of the general population [7]. The “graying” of the North America population and subsequent inevitable increases in organ failure and malignancy will continue to add to these numbers in the new millennium.
Listeriosis also continues to be a problem in veterinary medicine, causing epidemic abortion and encephalitis in ruminants. This encephalitis has been called “circling disease” because the infection occurs in the hindbrain and leads to ataxia in affected animals before death [8]. It is interesting that rhombencephalitis is also characteristic of human CNS disease in immunocompetent hosts. Cutaneous listeriosis may also be a potential hazard for veterinarians working with infected animals [9].
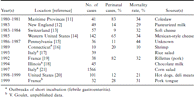
For many years, rhombencephalitis in animals had been associated with ingestion of spoiled silage contaminated by L. monocytogenes [10]. This observation from veterinary medicine led to speculation among epidemiologists that foodborne infection could be a cause of human listeriosis as well. This theory was finally proven correct by the analysis of an outbreak of listeriosis in humans caused by ingestion of contaminated coleslaw in the Maritime Provinces of Canada in 1981 [11]. Subsequently, several large outbreaks of human listeriosis have been identified (V. Goulet, unpublished data; [12–20]) that implicated a wide variety of contaminated foodstuffs, including meats, dairy products, and other processed foods meant to be consumed without further heating or pasteurization (table 1). The ability of this psychrotropic organism to proliferate at refrigerator temperatures provides a unique advantage over other pathogenic foodborne bacterial species in this environment, whose proliferation is inhibited by refrigeration.
Summary of data on major folklore outbreaks of Listeria monocytogenes infections, 1980–1999.
Epidemiology of L. monocytogenes Infection in Humans
Listeriosis remains an uncommon infection. Where active surveillance of sepsis and meningitis has been carried out, attack rates of listeriosis are ∼0.7 case per 100,000 population [22]. The infection is much more common in infants (10 cases per 100,000 population) and the elderly (1.4 cases per 100,000 population) and has a predominance among males. Infants acquire the infection in 2 ways. Mothers who are colonized in the gastrointestinal tract after eating contaminated food can develop occult sepsis resulting in chorioamnionitis and delivery of a septic infant or fetus. Alternatively, mothers carrying Listeria in the gastrointestinal tract and the perianal region may contaminate the skin and respiratory tract of their babies during childbirth. These infants can then develop bacterial meningitis up to 2 or 3 weeks after exposure at the time of birth. In North America, L. monocytogenes is the third most common pathogen causing bacterial meningitis among neonates, after group B streptococcus and Escherichia coli [23].
After the neonatal period, L. monocytogenes meningitis or sepsis is quite rare. Host factors that increase the risk of listerial infections include acquired and induced immunosuppression associated with HIV infection, solid organ transplantation, chemotherapy for solid and hematologic malignancies, hemochromatosis, diabetes mellitus, cirrhosis, and renal failure with hemodialysis or peritoneal dialysis [24]. Patients treated with fludarabine for chronic lymphocytic leukemia appear to be at greater risk of listeriosis than are patients treated with other chemotherapy regimens, perhaps because of a specific effect on T cell-mediated immunity [25].
In epidemic disease, different forms of listeriosis can occur. Some outbreaks have predominantly involved hospitalized patients with the risk factors noted above [26]. One neonatal outbreak in Costa Rica, however, was attributed to contaminated mineral oil used to clean infants after delivery [27]. Community-based outbreaks have most often involved large numbers of pregnant women giving birth to infected infants coupled with a few cases in healthy or immunocompromised adults. Other community-based outbreaks appear to involve equal numbers of mothers and immunocompromised adults in the community.
Since 1981, several large outbreaks of listeriosis have been described, and sources in food have been determined for each (table 1). The particular source foods were associated epidemiologically in each instance, and the implicated strain of Listeria was found in the food that was determined to be the source. In addition, active surveillance for Listeria, linked with laboratory studies of the refrigerator contents of patients with the disease, has proven that sporadic cases of listeriosis in the community also have sources in food [28, 29]. Strains of L. monocytogenes found in the refrigerators of patients were identical to strains isolated from the blood or CSF of the patient. Undercooked chicken and hot dogs appear to be frequent sources of infection, as are delicatessen meats and unpasteurized cheese products, especially soft cheeses.
As new techniques to identify Listeria in food have been developed and food inspection in search of microbial hazards has been strengthened, Listeria has been found in a wide variety of food products unassociated with any clinical disease. Therefore, L. monocytogenes must be a common transient colonizer of the human gastrointestinal tract but with little inclination to cause invasive infection unless host factors for invasive disease are present, or the amount delivered to the intestinal tract is large enough to overwhelm local gastrointestinal preventive barriers. Fecal surveys in the community that use selective media to identify and isolate Listeria demonstrate that the point prevalence of Listeria colonization is around 2%–10%, which may increase during an outbreak.
Clinical and Laboratory Features of Listeriosis
Neonatal infection. Two types of illness have been described [30]. “Early-onset” listeriosis develops from maternal sepsis and chorioamnionitis. This type of listeriosis can result in abortion, stillbirth, or premature delivery of a severely affected infant. It is interesting that twin pregnancies may be associated with a higher risk than are singleton births. Pustular skin lesions are characteristic of this syndrome, which has been termed “granulomatosis infantiseptica” [4] because of the granulomatous hepatitis that can often be found at autopsy in this illness. The mortality rate for infants born alive approaches 20%, and the frequency of abortion and stillbirth increases the overall mortality rate to >50%. The organism can be found in the infant's blood and CSF, in the placenta, and on the infant's skin, and the primary clinical picture is severe sepsis with multiorgan involvement.
On the other hand, “late-onset” neonatal listeriosis has the typical features of neonatal meningitis and occurs 7–20 days after delivery [31]. Infants with this type of listeriosis present with irritability and poor feeding and have physical signs of meningeal irritation. Gram staining and culture of CSF are usually positive (90%–95% of cases) and accompanied by an elevated WBC count and protein level and a decrease in the glucose concentration. The mortality rate associated with late-onset disease is ∼10%, but there may be residual neurological damage, as with other forms of neonatal bacterial meningitis.
Infections in adults. Mothers who deliver infants with early-onset listeriosis often have nonspecific flulike symptoms occasionally misdiagnosed as influenza or pyelonephritis [30]. A blood culture performed before the onset of labor that is positive for Listeria provides the opportunity to treat the infant in utero, but this opportunity is frequently missed because cultures may not be performed or results are delayed [14]. If left untreated, maternal listeriosis resolves spontaneously after delivery, while the infant remains critically ill.
Immunocompromised adults who develop bacterial meningitis have an acute (75%) or subacute (25%) presentation. Quite commonly however (40%–50% of cases), the clinical presentation is that of nonspecific bacteremia [24]. Listeriosis also can present as focal infection in a wide variety of organ systems, with or without accompanying sepsis. A list of clinical syndromes that have been attributed to L. monocytogenes infection, at least in anecdotal reports, is provided in table 2. Although the sources of infection have been documented for only a few of these syndromes, they are likely to have been food and to have developed after translocation of L. monocytogenes from the gastrointestinal tract.
Clinical syndromes described for Listeria monocytogenes infection.
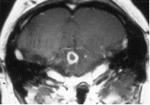
Rhombencephalitis due to Listeria has unique features and is commonly described in immunocompetent adults from a community where listeriosis is epidemic. In one series, the usual predisposing factors were present only in 30% of rhombencephalitis cases [32]. Patients with listerial meningoencephalitis have a subacute onset of illness that is characterized by focal neurological findings in the hindbrain, including ataxia and multiple cranial nerve abnormalities. Fever may be absent in 15% of cases, and alternate diagnoses are frequently entertained, including posterior fossa hemorrhage or stroke, metastatic malignancy, or subacute demyelinating disorders. CT or MRI scans usually demonstrate multiple microabscesses in the cerebellum and brain stem (figure 1), which are quite typical of the disorder. Analysis of CSF obtained by lumbar puncture shows an elevated protein level and modestly elevated mononuclear cell counts; Gram staining of CSF is usually negative. CSF cultures positive for Listeria may develop late, and blood cultures may demonstrate the organism first. Unfortunately, the confusing clinical picture and late identification of the organism, particularly in the absence of fever, may lead to late and ineffective treatment.
Enhancing microabscess in the brain stem of a 72-year-old man who presented with ataxia, cranial nerve palsies, and fever. Blood culture yielded Listeria monocytogenes.
Listerial endocarditis deserves specific mention because it often presents as prosthetic valve, rather than native valve, endocarditis [33]. Relapse is frequent, even in patients who have received standard treatment, and the mortality rate for patients with listerial endocarditis (48%) appears to be higher than the rate for patients with endocarditis due to other causes. This higher rate may be due to a lack of good antibiotic regimens that are bactericidal for Listeria.
Although invasive listeriosis remains the most common clinical syndrome identified in humans, several recent outbreaks of febrile gastroenteritis [16–18, 21] have demonstrated that L. monocytogenes, like Salmonella or Campylobacter species, can cause typical foodborne gastroenteritis. These outbreaks suggest that a large quantity of Listeria delivered to the gastrointestinal tract of healthy individuals can cause fever and diarrhea of rapid onset and limited duration. Patients become ill within 24–48 hours of exposure to the contaminated food; to date, outbreaks have demonstrated that rice salad, shrimp, chocolate milk, and corn salad are vehicles. Although vomiting can occur, diarrhea is almost universal in these patients and is not bloody. In a few instances, sepsis has occurred and caused increased morbidity and occasional mortality. The organism can be identified by using selective media, but laboratory screening of stool specimens for Listeria is recommended only when routine stool cultures are negative in the setting of an outbreak of gastroenteritis.
Treatment of Listeriosis
Untreated invasive listeriosis infection is fatal, except for pregnant women who deliver infants with early-onset listeriosis; such women clear their infection spontaneously after delivery. It is interesting that spontaneous resolution of invasive listeriosis also occurs in experimental animal models; some strains of rats and mice are intrinsically resistant to infection, while others develop sepsis and die following iv or ip inoculation with Listeria [34].
In vitro data and in vivo clinical experience suggest that a combination of ampicillin and an aminoglycoside is the favored treatment of invasive listeriosis [35]. Ampicillin is bacteriostatic for L. monocytogenes, and relapsing infection has been reported even with combination therapy. All strains of L. monocytogenes are uniformly resistant to cephalosporin antibiotics. Third-generation cephalosporins are commonly used in the empirical treatment of bacterial meningitis, but they must be combined with ampicillin when listerial meningitis or meningoencephalitis is suspected.
Vancomycin in combination with an aminoglycoside has been successfully used as treatment for penicillin-allergic patients with listeriosis. Another regimen with precedence in the literature is the combination of trimethoprim-sulfamethoxazole (TMP-SMZ) and rifampin. A French study [36] has suggested that this regimen is superior to ampicillin and aminoglycoside therapy, but the suggestion to use this combination as a first choice has not been widely accepted. The rarity of the illness makes the development of randomized, controlled trials of therapy impractical.
The generally accepted standard for duration of therapy is 3 weeks. For profoundly and irreparably immunocompromised patients, lifelong suppressive therapy may be necessary to prevent relapses. This treatment has been typically advocated for patients with advanced HIV infection who develop listerial meningitis.
Prevention of Listeriosis
Foodborne listeriosis can be prevented in 3 ways—by control of the organism in the food processing environment, by careful attention to preparation and choice of foods in the household, and, in specialized circumstances, by antibiotic prophylaxis. First, Listeria is widespread in the environment and readily introduced into abattoirs and other food processing plants. The organism proliferates in biofilms and in the low-temperature environment often found in these plants. Introduction into processed food is therefore inevitable, but contamination can be reduced by meticulous attention to principles of industrial hygiene. The food industry has introduced a program entitled Hazard Analysis at Critical Control Points to improve control of Listeria and other foodborne pathogens in these environments. This initiative has been associated with a reduction in sporadic disease in geographic regions where active surveillance for listeriosis is undertaken [37]. Despite this improvement, the US Food and Drug Administration continues to mandate “zero tolerance” for Listeria in food, and multiple recalls continue to take place as the number of food inspections increases, even in the absence of outbreaks of human disease. In other countries, contamination of food with small amounts of L. monocytogenes is allowed, and no data exist that show higher rates of human infection in these places.
Second, the large foodborne outbreaks of listeriosis have led to the publication of dietary recommendations for populations at risk [38] (table 3). These recommendations, and general public awareness of the problem of listeriosis, may have also contributed to decreases in sporadic cases of infection. However, foodborne outbreaks continue to occur against a background of sporadic disease, and there is little likelihood of eliminating the organism from the food supply completely and thereby preventing the disease in its entirety. Cooking or pasteurizing all foods would eliminate the risk of foodborne listeriosis entirely, but modern food preferences emphasize the “wholesomeness” of raw and minimally processed foods as part of a normal diet. The recent introduction of meat irradiation was met by public suspicion, but may lead to decreases in the incidence of listeriosis and other foodborne diseases.
Third, prophylactic antibiotic therapy may prevent some cases of listeriosis. The usefulness of treating pregnant women with ampicillin who have nonspecific febrile illness in the setting of a community-wide outbreak of listeriosis has already been mentioned. Patients with advanced HIV infection are commonly treated with TMP-SMZ daily or thrice weekly to prevent Pneumocystis carinii pneumonia. Surveillance data suggest that this treatment may be an effective preventive measure for listeriosis in this patient population as well [39]. Finally, some patients undergoing chemotherapy for malignancy may also benefit from prophylactic antibacterials, although the widespread use of quinolones in this setting may not be as effective against listeriosis as is TMP-SMZ prophylaxis.







Comments