-
PDF
- Split View
-
Views
-
Cite
Cite
Hilmar Wisplinghoff, Michael B. Edmond, Michael A. Pfaller, Ronald N. Jones, Richard P. Wenzel, Harald Seifert, Nosocomial Bloodstream Infections Caused by Acinetobacter Species in United States Hospitals: Clinical Features, Molecular Epidemiology, and Antimicrobial Susceptibility, Clinical Infectious Diseases, Volume 31, Issue 3, September 2000, Pages 690–697, https://doi.org/10.1086/314040
Close - Share Icon Share
Abstract
We examined the clinical and epidemiological features of nosocomial bloodstream infections (BSIs) caused by Acinetobacter species and observed from 1 March 1995 through 28 February 1998 at 49 United States hospitals (SCOPE National Surveillance Program). Acinetobacter species were found in 24 hospitals (49%) and accounted for 1.5% of all nosocomial BSIs reported. One hundred twenty-nine isolates were identified either as A. baumannii (n = 111) or other Acinetobacter species (n = 18). Patients with A. baumannii BSI, compared with patients with nosocomial BSI caused by other gram-negative pathogens, were more frequently observed in the intensive care unit (69% vs. 47%, respectively; P < .001; odds ratio [OR] 2.4; 95% confidence interval [CI] 1.6–3.7) and were more frequently receiving mechanical ventilation (58% vs. 30%, respectively; P < .001; OR 3.2; 95% CI 2.1–4.8). Crude mortality in patients with A. baumannii BSI was 32%. Molecular relatedness of strains was studied by use of polymerase chain reaction-based fingerprinting. Clonal spread of a single strain occurred in 5 hospitals. Interhospital spread of epidemic A. baumannii strains was not observed. The most active antimicrobial agents against A. baumannii (90% minimum inhibitory concentration values) were imipenem (1 mg/L; 100% of isolates susceptible), amikacin (8 mg/L; 96%), tobramycin (4 mg/L; 92%), and doxycycline (4 mg/L; 91%). Thirty percent of isolates were resistant to ≥4 classes of antimicrobials and were considered to be multidrug resistant.
Acinetobacter species are a heterogenous group of organisms that have emerged as significant nosocomial pathogens mainly affecting patients with impaired host defenses in the intensive care unit (ICU) setting. Members of the genus Acinetobacter, particularly A. baumannii, are implicated in a wide spectrum of infections, including nosocomial pneumonia, secondary meningitis, skin and soft tissue infections, urinary tract infections, and bacteremia [1].
The crude mortality of A. baumannii bloodstream infection (BSI) may be as high as 52% [2–4]. Outbreaks of infections are often associated with the spread of a unique strain and have been linked to contaminated respiratory therapy equipment [5], intravascular access devices [2], bedding materials [6, 7], and transmission via the hands of hospital personnel [8]. The persistence of endemic A. baumannii strains in the ICU seems to be related to their ability for long-term survival on inanimate surfaces in the patients' immediate environment [9] as well as to their widespread resistance to the major antimicrobial agents [10, 11]. European investigators have recently suggested that—similar to the epidemiology of methicillin-resistant Staphylococcus aureus— a few epidemic A. baumannii strains may be involved in outbreaks at various institutions as well as in international spread [12–15].
The purpose of this study was to investigate the predisposing factors for and the clinical and microbiological features of Acinetobacter BSIs reported from hospitals throughout the United States. The frequency of epidemic strains and the possible impact of interhospital spread of these organisms were studied by molecular typing with randomly amplified polymorphic DNA (RAPD) analysis.
Material and Methods
Background. The Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) project, situated at the Medical College of Virginia in Richmond, was established to identify the predominant pathogens and antimicrobial susceptibilities of nosocomial bloodstream isolates. The 49 participating hospitals throughout the United States represent medical institutions of various sizes (range, 60–1200 beds) from a broad range of geographical regions [16, 17]. Data generated by the SCOPE Project have shown a high correlation with data from the National Nosocomial Infection Surveillance program of the Centers for Disease Control and Prevention (CDC) [16].
Study design. Clinical data and bloodstream isolates were prospectively collected from patients admitted, from 1 March 1995 through 28 February 1998, to 1 of 49 hospitals participating in the SCOPE project. The patient data that were collected included age, sex, underlying diseases, risk factors, and sources of BSI. Nosocomial BSI was determined by use of standardized criteria, and the patient had to have had ≥1 positive blood culture while meeting the CDC definition for nosocomial BSI [17, 18]. Sources of secondary BSI were identified by cultures obtained from distant sites that yielded the same pathogen with an identical resistance pattern.
For the purpose of this study, all patients with BSI caused by Acinetobacter species and whose blood culture isolates were available were included. Multiple blood cultures yielding the same Acinetobacter species were considered to be a single infection. Clinical characteristics, underlying diseases, possible risk factors, and outcomes of patients with Acinetobacter BSI were compared with those of 2952 patients who were admitted during the study period and who had nosocomial BSI caused by other gram-negative aerobic bacteria.
Microbiology. Blood cultures were processed at the participating hospitals. Primary identification of blood isolates and susceptibility testing were done using the routine methods in use at the affiliated laboratories. On a monthly basis, isolates were sent to the microbiology laboratories at the University of Iowa, Iowa City, and to the Medical College of Virginia at Virginia Commonwealth University, Richmond, respectively, for storage and further characterization. Acinetobacter isolates were frozen at −70°C until they were forwarded to the Institute of Medical Microbiology, Immunology and Hygiene, University of Cologne, Germany, for further investigation. Identification of Acinetobacter species at the genus level was confirmed by use of the transformation assay as described by Juni [19]. Speciation was performed with the simplified identification scheme of Bouvet and Grimont [20], which included growth at 37°C, 41°C, and 44°C; production of acid from glucose; gelatin hydrolysis; and use of 14 different carbon sources.
Strain relatedness of all isolates was assessed as described elsewhere [21], with RAPD-PCR analysis performed with 2 different primers (primers ERIC-2 and M13) with Ready-to-Go RAPD Analysis beads (Pharmacia Biotech, Freiburg, Germany). These beads contain thermostable polymerases (AmpliTaq and Stoffel fragment), lyophilized buffer, deoxynucleoside-5′-triposphate (dNTPs), and bovine serum albumin. An internal A. baumannii control strain was run in 3–4 lanes per gel. Amplified fragments were separated by agarose gel electrophoresis and were visualized under ultraviolet illumination after staining with ethidium bromide. Fingerprint patterns were photographed and compared both visually and by computer-aided analysis with use of Molecular Analyst software (Bio-Rad Laboratories, Munich, Germany).
MICs to selected antimicrobial agents were determined by the reference broth microdilution method as described by the National Committee for Clinical Laboratory Standards [22], with use of a commercially manufactured plate (Micronaut-S, Merlin Diagnostics, Bornheim, Germany). Copy strains with an identical PCR fingerprint pattern were excluded even if they had been isolated from different patients. The following antimicrobial agents were included: ampicillin, piperacillin, cefazolin, cefuroxime, cefoxitin, cefotaxime, cefepime, ceftazidime, imipenem, gentamicin, amikacin, tobramycin, ciprofloxacin, levofloxacin, trovafloxacin, doxycycline, and cotrimoxazole (trimethoprim/sulfamethoxazole). Plates were read after incubation at 35°C for 20–24 h in ambient atmosphere. The MIC was defined as the lowest concentration of drug that prevented visible growth. S. aureus ATCC 25923, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853 were used as controls. Interpretative criteria for each antimicrobial tested were those published by the National Committee for Clinical Laboratory Standards [22].
Statistical analysis. Results were expressed as the mean ± SD or as a proportion of the total number of patients. Univariate analysis was conducted by use of the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables. The α value was set at P ≤ .05, and probability values are 2-tailed. All statistical analyses were done with the SPSS software package (Chicago, IL).
Results
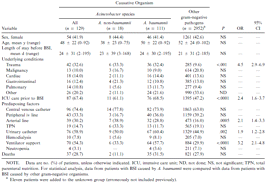
Study population and patient characteristics. From 1 March 1995 through 28 February 1998, a total of 10,852 cases of BSI were reported by hospitals participating in the SCOPE project. Among these, 166 clinically significant episodes of bacteremia caused by Acinetobacter species were identified in 24 (49%) of 49 hospitals. Among 152 adult and 14 pediatric patients, these infections accounted for 1.5% of all nosocomial BSIs reported. No seasonal or geographical patterns were observed. Further analysis was limited to those episodes (n = 129) for which Acinetobacter blood isolates were available for further investigation. Patient characteristics and predisposing factors that were present within 48 h prior to episodes of bacteremia are shown in table 1. Slightly more than half of the patients with BSI caused by Acinetobacter species were men (n = 75; 58%). Patients had a mean age of 48 ± 22 years (range, 0–92 years). Length of stay prior to BSI averaged 24 ± 31 days (range, 2–195 days).
Clinical characteristics and predisposing factors of patients with bloodstream infections (BSIs) caused by either Acinetobacter baumannii, Acinetobacter species other than A. baumannii, or other gram-negative pathogens.
Underlying conditions included trauma (n = 42; 33%) and malignancies (n = 13; 10%) as well as cardiac (n = 18; 14%), gastrointestinal (n = 16; 12%), and pulmonary diseases (n = 14; 11%). ICU care was required by 87 patients (67%) before BSI. Ventilator support and total parenteral nutrition were used in 70 (54%) and 19 (15%) of the patients, respectively. Central venous catheters were present in 96 patients (74%), and peripheral intravenous and arterial lines were present in 43 (33%) and 39 (30%) patients, respectively. Urinary catheters were present in 76 patients (59%).
When patients with BSI caused by A. baumannii (n = 111) were compared with patients with BSI caused by other gram-negative pathogens (n = 2952), no significant differences with regard to the distribution of age or sex were observed. Trauma as a predisposing condition was more prevalent among patients with A. baumannii BSI (32% vs. 10%; P < .001; OR, 4.5; 95% CI, 2.9–6.9). Pulmonary and cardiac diseases were also more frequently seen in these patients, whereas malignant conditions were more prevalent among patients with BSI caused by other gram-negative pathogens. However, none of these differences reached statistical significance. Before BSI, patients with A. baumannii BSI, compared with control patients, were more frequently hospitalized in the ICU (69% vs. 47%; P < .001; OR, 2.4; 95% CI, 1.6–3.7) and were more frequently receiving mechanical ventilation (58% vs. 30%; P < .001; OR, 3.2; 95% CI, 2.1–4.8). Other risk factors were almost equally distributed in both groups, with intravascular devices and urinary catheters being more prevalent in patients with A. baumannii, whereas total parenteral nutrition and neutropenia (absolute neutrophil count, <1000 cells/µL) were more frequently observed among control patients, without reaching statistical significance. Overall mortality was slightly higher in patients with A. baumannii BSI than in control patients (32% vs. 28%; P = .38, NS).
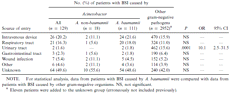
Origin of bacteremia. Sources of A. baumannii BSI were identified in 57 patients (table 2). Intravenous lines were the most frequently detected portal of entry (n = 24; 22%), followed by the respiratory tract (n = 20; 18%) and contaminated wounds (n = 5; 4.5%). A portal of entry could not be determined in 54 patients (49%), in part because of the lack of cultures taken from distant sites. A. baumannii BSI, compared with BSI caused by with other gram-negative organisms, originated only rarely from the urinary tract (2% vs. 16%; P < .001; OR, 10.1; 95% CI, 2.5–31.5) or from a gastrointestinal source (2% vs. 6%; P = .07, NS). Polymicrobial BSI was seen in 46 (36%) of 129 patients. Coagulase-negative staphylococci (35%) and enterococci (21%) were most frequently isolated concomitantly. There were no differences in outcome between patients with monomicrobial and polymicrobial BSI.
Sources of bloodstream infection (BSI) caused by either Acinetobacter baumannii, Acinetobacter species other than A. baumannii, or other gram-negative pathogens.
Species identification and epidemiological typing. Among Acinetobacter species isolated from blood cultures during the study period (n = 166), 129 isolates were available for further characterization. Of the remaining 37 isolates, 33 had not been sent to the study center, and 4 were no longer viable. The 129 study isolates were identified to the species level, and the findings were as follows: A. baumannii, n = 111 isolates; Acinetobacter DNA group 3, n = 11; Acinetobacter radioresistens, n = 4; Acinetobacter junii, n = 2; and Acinetobacter DNA group 11, n = 1. These isolates had originally been identified, at their respective study centers: A. baumannii, 84 isolates (65%); Acinetobacter calcoaceticus, 21 (16%); Acinetobacter anitratus, 18 (14%); and Acinetobacter lwoffii, 2 (1.5%). Four isolates had not been speciated previously.
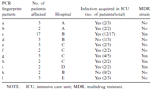
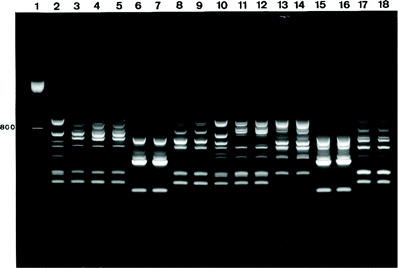
RAPD-PCR typing of A. baumannii isolates with both M13 and ERIC-2 primers resulted in 78 different fingerprint patterns. These included 67 unique patterns (60%) seen in only 1 patient. Two or more isolates exhibiting the same fingerprint pattern were observed in 5 (24%) of 21 hospitals, thus suggesting nosocomial cross-transmission of A. baumannii. For the purpose of this study, these strains were considered epidemic strains. Two major epidemic A. baumannii strains were observed in 2 different hospitals and were involved in nosocomial BSI observed in 17 and 5 patients, respectively. Nine other strain types affected 2 or 3 patients each (table 3). Representative fingerprint patterns are shown in figure 1. Nine (82%) of 11 epidemic strains were recovered from patients in the ICU. A particular banding pattern that was shared by 2 isolates obtained from patients hospitalized in different hospitals was not observed. Therefore, there was no evidence for interhospital spread of major epidemic A. baumannii strains. All Acinetobacter isolates other than A. baumannii that were isolated from different patients exhibited unique fingerprint patterns.
Distribution of epidemic Acinetobacter baumannii strains in 5 United States hospitals.
Randomly amplified polymorphic DNA fingerprint patterns obtained for Acinetobacter baumannii blood isolates after PCR amplification with M13 primer showing representative isolates of the following different A. baumannii strain types: types a (lanes 3–5), b (lanes 6 and 7), c (lanes 8 and 9), d (lanes 11 and 12), e (lanes 13 and 14), f (lanes 15 and 16), and g (lanes 17 and 18). Lane 1, 100-bp ladder. Lanes 2 and 10, internal A. baumannii reference strain. For origin of strains, see table 3.
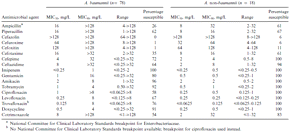
Susceptibility testing.Table 4 lists the results for 17 antimicrobial agents tested against Acinetobacter species. A. baumannii isolates (78 isolates with different fingerprints) were generally more resistant than Acinetobacter isolates identified as species other than A. baumannii. Against A. baumannii, the most active agents, as shown by their 90% MIC values, were imipenem, amikacin, tobramycin, and doxycycline. No strain resistant to imipenem was found. Among the aminoglycosides, gentamicin was the least active agent, with 80% of isolates being susceptible. Susceptibility rates were even higher for tobramycin (92%) and amikacin (96%). Doxycycline was also very active and inhibited 91% of isolates. Among the quinolones, ciprofloxacin was less active (58% susceptible) than levofloxacin or trovafloxacin (67% and 76% of isolates susceptible, respectively). Among the β-lactams, only piperacillin, ceftazidime, and cefepime showed moderate activity, with 62%, 64%, and 72% of isolates, respectively, showing susceptibility. Multidrug resistance, which was defined by resistance to ≥4 classes of antimicrobial agents (i.e., β-lactams, aminoglycosides, quinolones, cotrimoxazole, doxycycline, and imipenem), was noted in 33 isolates (30%) representing 9 different strain types (12%). Among these were 4 epidemic strains of multidrug-resistant organisms that affected 2, 2, 5, and 17 patients, respectively. Another 5 multidrug-resistant A. baumannii strains were seen in only 1 patient.
In vitro activities of 17 antimicrobial agents against isolates of Acinetobacter baumannii and species of Acinetobacter other than baumannii.
Discussion
In recent years, Acinetobacter species, mainly A. baumannii, have assumed increasing importance as nosocomial pathogens, most commonly among patients in the ICU. The characteristics of Acinetobacter bacteremia have been described by researchers from various parts of the world [3, 4, 23–26]. The present study focuses on A. baumannii BSI in the United States and includes 129 cases. To our knowledge, this is the largest series of Acinetobacter BSI reported to date, and it is the first to provide data from a large number of different medical centers. We could not observe a seasonal variation in the frequency of Acinetobacter BSI, a finding unlike those of other studies from the United States [27, 28].
To identify risk factors associated with the acquisition of A. baumannii BSI, we compared 111 patients with A. baumannii BSI with 2952 control patients with nosocomial BSI caused by other gram-negative pathogens. Patients with A. baumannii BSI tended to be more severely compromised before bacteremia than were the control patients. Although both patient groups did not differ in their prior length of hospitalization before bacteremia, patients with A. baumannii BSI were more frequently hospitalized in an ICU and were also more often receiving mechanical ventilation. Conversely, in a recent study from Hong Kong, Siau et al. [25] reported that only 22% of patients with A. baumannii BSI acquired their infection in the ICU. In the present study, trauma as an underlying condition was significantly more frequent in patients with A. baumannii BSI than in control patients, as were intravascular and urinary catheters. Unfortunately, previous antimicrobial therapy could not be evaluated as a potential risk factor, because these data were not obtained during the SCOPE project.
In a previous investigation performed on burn patients, we identified the extent of the underlying burn injury, previous colonization with A. baumannii at a distant site, and hydrotherapy significantly associated with A. baumannii BSI [26]. Other investigators have found various risk factors associated with A. baumannii colonization and infection, such as extended hospital stay, severity of illness markers, organ failure, mechanical ventilation, presence of intravascular access devices, and previous antibiotic therapy [4, 24, 29–32]. However, most researchers have studied the acquisition of A. baumannii in the setting of a nosocomial outbreak and have only rarely included a sufficient number of patients with BSI [8, 29–32].
The similar but slightly increased overall mortality rate among patients with A. baumannii BSI, compared with that in patients with BSI caused by other gram-negative organisms (32% vs. 28%, respectively), may simply reflect differences in the severity of the underlying illness. However, it underscores the clinical significance of these organisms that have often been regarded as harmless commensals or low-virulent pathogens [33, 34]. Our findings concur with previously reported crude mortality rates of A. baumannii BSI (range, 17%–52%) [2–4, 23, 34]. Again, A. baumannii BSI tended to run a milder clinical course in Hong Kong, with an overall mortality of only 11%; this is comparable to the low mortality associated with BSI caused by Acinetobacter species other than A. baumannii that was observed in the present study (11%) as well as in previously published reports [35].
Unfortunately, as in other studies [4, 23, 36], the source of A. baumannii bacteremia was not identified in 49% of our patients. Our finding that intravascular catheters and the respiratory tract were the most common portal of entry for A. baumannii BSI is in agreement with previous data. Several studies found the respiratory tract to be the most common source of Acinetobacter BSI [4, 23, 24]. Other researchers have emphasized the association of A. baumannii with catheter-related BSI [2, 3, 25, 37]. As in our study, the role of intravascular catheters in the pathogenesis of A. baumannii BSI may be underestimated if quantitative culturing of catheters is not performed and if clinical criteria for the diagnosis of catheter-related BSI, such as those proposed by Raad and Bodey [38], are not employed. It is of note that the urinary tract and the gastrointestinal tract do not seem to be an important source for A. baumannii BSI, because these sites were identified as a portal of entry for A. baumannii considerably less frequently than for BSI caused by other gram-negative pathogens.
A. baumannii accounted for 86% of all Acinetobacter isolates identified to species level, a finding that confirms previously reported data [1, 3, 23, 39]. Identification of Acinetobacter species according to current taxonomy had been performed in only 38% of laboratories. Thirty-five percent of isolates had been identified, on the basis of the old taxonomy of the genus, as A. calcoaceticus, A. anitratus, and A. lwoffii or had not been speciated previously.
The epidemiology of A. baumannii infections has been investigated in several studies in Europe, the United States, South America, and Asia [11, 12, 14, 21, 25, 31, 32, 40–42]. Investigations of hospital outbreaks suggest that exposure to colonized patients and contaminated medical equipment is associated with A. baumannii colonization and infection [5, 6, 8, 30]. For epidemiological studies of A. baumannii, various typing methods have been employed [12, 21, 41, 42]. Of these, RAPD-PCR is a highly reliable and reproducible method if standard protocols are used, and it permits rapid discrimination of epidemic and sporadic strains. In our study, the majority of BSIs were due to patient-unique A. baumannii strains. Clusters of A. baumannii BSI involving ≥3 patients were identified in 4 of the participating hospitals. A major outbreak of A. baumannii BSI involving 17 patients was observed in only 1 hospital.
Because we focused on BSI only, the design of this study did not allow us to investigate the mode of spread of A. baumannii in the participating hospitals, because no information was available with regard to the colonization and infection with A. baumannii at other body sites. In fact, the observation that 2 patients had documented A. baumannii BSI with the same strain and within a limited period of time—as was the case in 4 hospitals—suggests that patient-to-patient transmission has occurred and may even raise the suspicion that epidemic spread was involved. However, molecular analysis of blood culture isolates is certainly not sufficient to study the epidemiology of A. baumannii, and it may lead to underestimation of the impact of nosocomial cross-transmission of these organisms.
In recent years, several authors in Europe have claimed that geographical spread at a national or international level may be an important feature in the epidemiology of A. baumannii. Dijkshoorn et al. [13], who studied outbreak isolates from different medical centers in northwestern Europe with various typing methods, reported groups of strains that had been recovered from hospitals in The Netherlands and Denmark that were highly similar by at least 1 genomic and 1 other typing method, concluding that this finding may be explained by a common clonal origin of the strains involved. This finding was confirmed by Nemec et al. [14], who identified A. baumannii isolates from geographically distinct locations in the Czech Republic that shared a specific ribotype and were highly similar in other properties. Vila and colleagues [15] recently observed the spread of an epidemic amikacin-resistant A. baumannii strain in 9 different hospitals in Spain. In our series, we did not observe any interhospital spread of A. baumannii. No strains with an identical fingerprint pattern were found in more than 1 hospital. However, because only 49 medical centers across the United States were monitored, we cannot rule out the possibility that interhospital spread of A. baumannii strains occurs at least at a local or regional level. Our data from a national surveillance system do not support the concept that geographical spread of certain epidemic A. baumannii strains plays a major role in the epidemiology of A. baumannii. Further studies that include a larger number of hospitals and do not focus on BSI only will be needed to address this question in more detail.
Increased resistance among A. baumannii was generally observed in many recent studies [3, 11, 43–46]. However, because epidemiological relatedness of strains was not assessed in most of these studies, susceptibility data may have been influenced by the unrecognized inclusion of epidemic strains that are often multidrug resistant. On the basis of molecular typing data, we could ascertain that only A. baumannii strains with a distinct fingerprint pattern were included and that epidemic strains were represented by a single isolate only. We detected high rates of resistance to β-lactam antibiotics and quinolones in A. baumannii isolates throughout the United States. Imipenem resistance is a great concern, and resistant strains have been reported from various parts of the world, including the United States [4, 11, 40, 47, 48]. In our study, all isolates were susceptible to imipenem. Resistance rates of A. baumannii to fluoroquinolones varied considerably in recent studies. Chang et al. [45] found that 98% of Acinetobacter bloodstream isolates in Taiwan were susceptible to ciprofloxacin, a finding comparable to reports from the United States in the 1980s [49]. These data contrast with those from studies from Germany and Spain [4, 10, 43], where up to 97% of isolates were resistant to ciprofloxacin.
We found resistance to all quinolones tested in 23% of isolates, with resistance to ciprofloxacin in 37%. Compared with other recent studies [4, 10, 11, 15] with 60%–80% rates of resistance to aminoglycosides, resistance to these compounds in our series was low, with 80% of strains susceptible to gentamicin and >90% susceptible to amikacin and tobramycin. Multidrug resistance in A. baumannii strains has become a great concern over the past years [1, 3, 4, 11, 44]. We found 30% of all A. baumannii isolates, representing 9 different strain types, to be resistant to ≥4 different classes of antimicrobial agents. Among these were 4 outbreak strains affecting between 2 and 17 patients each. There was no more than 1 multidrug-resistant strain in a given hospital.
In summary, we have documented the contemporary frequency of A. baumannii among nosocomial blood stream isolates in the United States and have demonstrated that A. baumannii BSI is most commonly observed among patients hospitalized in ICUs. Associated mortality is high, as are resistance rates to various antimicrobial agents. The majority of nosocomial BSIs caused by Acinetobacter species originated from intravascular catheters and the respiratory tract. Patient-to-patient transmission appears to be a frequent mode of spread. However, there was no evidence for transmission of epidemic organisms between hospitals.
Acknowledgments
We wish to thank D. Stefanik, S. E. Wallace, S. Talbot, and D. K. McClish, for excellent technical assistance, and the infection-control practitioners and microbiology laboratory personnel at the participating hospitals, for their help.
References
Presented in part: 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 26–29 September 1999 (abstract K-1428).





Comments