-
PDF
- Split View
-
Views
-
Cite
Cite
ELISABET JERLHAG, MORTEN GRØTLI, KRISTINA LUTHMAN, LENNART SVENSSON, JÖRGEN A. ENGEL, ROLE OF THE SUBUNIT COMPOSITION OF CENTRAL NICOTINIC ACETYLCHOLINE RECEPTORS FOR THE STIMULATORY AND DOPAMINE-ENHANCING EFFECTS OF ETHANOL, Alcohol and Alcoholism, Volume 41, Issue 5, September/October 2006, Pages 486–493, https://doi.org/10.1093/alcalc/agl049
Close - Share Icon Share
Abstract
Aims: The stimulatory, rewarding, and dopamine (DA)-enhancing effects of ethanol may involve central nicotinic acetylcholine receptors (nAChR), especially those located in the ventral tegmental area (VTA). Identifying the subunit composition that mediates these effects of ethanol would increase the understanding of the neurochemical basis underlying the addictive properties of ethanol. In the present series of experiments, the role of the
(Received 22 December 2005; first review notified 20 January 2006; in revised form 12 May 2006; accepted 16 May 2006)
INTRODUCTION
Several clinical and epidemiological studies have demonstrated an association between the consumption of alcohol and the use of tobacco, i.e. nicotine, among the general population as well as among alcoholics (Istvan and Matarazzo, 1984; Bien and Burge, 1990; Miller and Gold, 1998). For instance ∼80–90% of all alcoholics are smokers (Walton, 1972; Ayers et al., 1976; Bien and Burge, 1990; Batel et al., 1995; Miller and Gold, 1998), smokers consume twice as much alcohol than non-smokers (Carmody et al., 1985), and alcoholism is estimated to be 10–14 times more common among smokers than non-smokers (DiFranza and Guerrera, 1990). There also appears to be a strong correlation between the onset of tobacco abuse at an early age and addiction to alcohol later in life (DiFranza and Guerrera, 1990; Grant, 1998). Moreover, ethanol has been found to potentiate the rewarding effects of nicotine among smokers (Rose et al., 2004). Taken together, these clinical findings suggest that ethanol and nicotine can share important neurochemical mechanisms of action in the brain reward systems, e.g. involving nicotinic acetylcholine receptors (nAChR). The first evidence for a possible interference between ethanol and nAChR was discovered in our laboratory showing that chronic ethanol consumption produced changes in the Bmax for [3H]nicotine in different regions of the rat brain (Yoshida et al., 1982). In subsequent studies on the functional significance of these findings we have found that ethanol intake and preference as well as ethanol-induced stimulation of the mesolimbic DA system and of locomotor activity may involve activation of central nAChR, especially those in the ventral tegmental area (VTA) (Larsson and Engel, 2004; Larsson et al., 2005). Further support for the assumption that the nAChR could serve as one common denominator for the ethanol-nicotine interaction has been provided from mainly electrophysiological studies showing that ethanol can stabilize the open state of the Torpedo nAChR (Wu et al., 1994; Forman and Zhou, 1999), increase the agonist affinity for this receptor (Forman et al., 1989), and enhance the response to nicotine indicating that ethanol may also act as a co-agonist to ACh on nAChRs (Marszalec et al., 1999).
The pentameric nAChRs are widely distributed in the brain and the mesocorticolimbic DA pathway expresses high levels of nAChRs, both pre-synaptic and somatodendritic (Clarke and Pert, 1985). In the central nervous system α2–α7, α9, α10, and β2–β4 subunits of the nAChR have been identified and these can be expressed in various combinations of either heteromeric or homomeric receptors (Nicke et al., 2004). The α3–α7 and β2–β4 (Klink et al., 2001) as well as the α3–α6 and β2–β3 (Charpantier et al., 1998; Le Novere et al., 2002) subunits of the nAChR have been identified in the VTA. The central nAChRs, especially those located in the VTA, might play a central role in the neurochemical events mediating the stimulatory, DA-enhancing, and rewarding properties not only of nicotine (Clarke et al., 1988; Wonnacott et al., 1990; Di Chiara, 2000) but also of ethanol (Blomqvist et al., 1997; Larsson et al., 2002; Ericsson et al., 2003). Since the subunit composition in all probability may influence the sensitivity to ethanol we have been investigating the role of these subunits in mediating the behavioural and neurochemical effects of ethanol.
Thus, we have previously found that neither dihydro-β-erythroidine (DHβE), selective for the
In our search for defining the nAChR subunits involved in the behavioural and neurochemical effects of ethanol we have turned to the peptide α-conotoxin PIA (αCtxPIA), which shows significantly higher selectivity for the
The amino acid sequences for α-conotoxin MII, α-conotoxin PIA, and the α-conotoxin PIA-analogue. The eight similar amino acids between the three peptides are highlighted.
Since the access to αCtxMII and αCtxPIA, as well as to other α-conotoxins, is limited the first aim of the present study was to synthesize the αCtx peptides and analogues using a modified synthetic procedure described for αCtxMII (Cartier et al., 1996). This procedure was used to synthesize α-conotoxin peptides with different nAChR selectivity than αCtxMII, e.g. αCtxPIA. The nAChR subunit selectivity of 4/7 α-conotoxins has been reported to reside to the central and the C-terminal part of the peptide, whereas the N-terminal amino acids appear to be of less importance (McIntosh et al., 1999; Arias and Blanton, 2000; Dutertre and Lewis, 2004; Everhart et al., 2004; Dutertre et al., 2005). Specifically, the bulky charged N-terminal protrusion of αCtxPIA has been suggested to be of less importance for its interaction with the α6 subunit of the nAChR (Chi et al., 2005). We therefore synthesized the analogue to αCtxPIA, αCtxPIA-analogue, where the first amino acid, i.e. arginine, in the N-terminal part was removed (Fig. 1). To maintain the selectivity to the
The effects of synthesized αCtxMII and the αCtxPIA-analogue on ethanol-induced locomotor stimulation in mice, as well as the effects of the synthesized αCtxPIA-analogue on ethanol-induced DA-overflow in N.Acc. in awake freely moving mice, were also investigated.
MATERIALS AND METHODS
Peptide synthesis
Linear peptides
The peptides were synthesized on a Rink amide resin (loading 1.20 mmol/g) using N-9-fluorenylmethoxycarboxyl (Fmoc) chemistry and O-(7-benzotriazole-1-yl)-1,1,3,3 tetramethyluronium tetrafluoroborate (TBTU) and N,N-diisopropylethyl amine (DIPEA) activation. The peptides were synthesized on a 0.24 mmol scale with standard amino acid side chain protection (Glu and Ser [t-Bu]; Asn and His [trityl] except on cysteine residues). Cysteine residues were protected in pairs with S-trityl on the first and third cysteines and S-acetamidomethyl on the second and fourth cysteines. Each residue was used in 5-fold excess and coupled for 60 min.
After the synthesis was completed the resin was washed with methanol (3 × 10 ml) and dried under reduced pressure.
The linear peptide amide was cleaved of the resin by treatment with 6 ml of trifluoroacetic acid/H2O/ethanedithiol/thioanisole (94.5/2.5/2.5/1 by volume) for 6 h at 20°C. The resin was rinsed twice with 6 ml of the same solution. The combined filtrate and washings were pooled and concentrated to dryness. The residue was washed two times with diethyl ether. The supernatant was discarded and the precipitated peptide powder was dried on the vacuum line.
Peptide cyclization
To form a disulfide bridge between Cys and Cys (i.e. the first and third cysteines), the pelleted peptide was dissolved in 1.2 ml of H2O/acetonitrile/trifluoroacetic acid (95/5/0.1 by volume)/H2O (40/60 by volume), with gentle swirling (to avoid foaming). The linear peptide solution was added dropwise into 57 ml of H2O (pH 7.6 by solid Tris base). The solution was gently swirled at room temperature for 45 h when the reaction was judged to be complete by analytical HPLC using a Genesis C18 column (4μ, 15 cm × 4.6 mm; Jones Chromatography, Hengoed, USA) and a gradient from 5 to 95% of B-Buffer [H2O/acetonitrile/trifluoroacetic acid (95:5:0.1 by volume)] in acetonitrile as eluent (flow rate at 1 ml/min). The pH of the solution was adjusted to 2–3 by the addition of trifluoroacetic acid and the solution was freeze-dried. The monocyclic peptide was then purified by preparative HPLC using an Ace 5AQ column (25 cm × 21.2 mm; Advanced Chromatography Technologies, Aberdeen, UK) and a gradient from 5 to 95% of B-Buffer in acetonitrile as eluent (flow rate at 10 ml/min). The fractions containing the product were pooled and freeze-dried.
Removal of the S-acetamidomethyl groups and formation of the second disulfide bridge (Cys-Cys, i.e. the second and fourth cysteines) were carried out simultaneously by iodine oxidation. The monocyclic peptide was diluted in 3.5 ml of B-Buffer and added dropwise to 3.5 ml of a rapidly stirred solution of 20 mM iodine in H2O/trifluoroacetic acid/acetonitrile/MeOH (50:20:20:10 by volume) over 5.5 min at room temperature. This reaction was allowed to proceed for another 90 min and was thereafter terminated by the addition of a diluted aqueous solution of ascorbic acid (1 M, two drops). The solution was freeze-dried over night and the peptide was obtained as a powder. The peptide was dissolved in 450 μl of B-Buffer and was purified by preparative HPLC as described above. The purity of the peptide was analysed by analytical HPLC as described above. The peptide was thereafter freeze-dried and the amino acid sequence was analysed by FAB-MS (Einar Nilsson, Department of Organic Chemistry, Lund University, Sweden). The peptide was thereafter used in the animal experiment.
Animals
Male adult NMRI mice weighing ∼30–40 g, purchased from Charles River (Sulzfeld, Germany), were used. They were housed in groups of eight in standard plastic cages (Macrolon III; 400 × 250 × 150 mm) with free access to standard laboratory food (Harlan Teklad; Norfolk, England) and tap water. Upon arrival, the mice were allowed to habituate to the animal facilities for at least 1 week. A temperature of 20°C, humidity of 50%, and a 12/12 h light/dark cycle (lights switched on at 7:00 a.m.) were maintained in the animal room. The present study was approved by the Ethics Committee for Animal Experiments, Göteborg, Sweden.
Locomotor activity procedure
Surgical procedure
Since αCtxMII and the αCtxPIA-analogue might not pass the blood–brain barrier they were administered locally and bilaterally into the VTA. To facilitate this administration two bilateral guide cannulas, aiming at the VTA, were surgically implanted four days prior to the experiment. The mice were anesthetized with isoflurane (Isofluran Baxter; Univentor 400 Anaesthesia Unit, Univentor Ltd, Zejtun, Malta), placed in a Kopf stereotaxic instrument (David Kopf Instruments, Tujunga, CA, USA) and kept on a body temperature-regulating heating pad to prevent hypothermia. The skull bone was exposed and two holes for the guide cannulas (stainless steel, length 10 mm, with an o.d./i.d. of 0.6/0.45 mm) and one for an anchoring screw were drilled. The coordinates for the VTA relative to the bregma were posterior −3.4 mm, lateral to midline ±0.5 mm, and ventral −1.0 mm from the brain surface (Franklin and Paxinos, 1996). The guide cannulas and the anchoring screw were stabilized with dental cement (Dentalon Plus; AgnThós AB, Lidingö, Sweden). After surgery the mice were kept in individual cages (Macrolon III) with food and water supplies ad libitum and were allowed to recover for 4 days before the locomotor activity testing. At time of the experiment a cannula for drug administration was inserted and extended another 3.8 mm ventrally beyond the tip of the guide cannula (Fig. 2).
Coronal mouse brain section showing probe placements in the nucleus accumbens and VTA. Coronal mouse brain section showing probe placements (illustrated by vertical lines) in the nucleus accumbens or within the VTA of 10 representative mice used in the present study. The number in the forebrain section indicates millimetres from bregma. +1.5 mm; nucleus accumbens. −3.4 mm; VTA. Reprinted from The Mouse Brain in Stereotaxic Coordinates, K. B. J. Franklin and G. Paxinos, figure 18, Copyright (1996), with permission from Elsevier.
Locomotor activity measuring device
Locomotor activity was registered in eight sound attenuated, ventilated, and dim lighted locomotor boxes (420 × 420 × 200 mm; Plexiglas®). Five by five rows of photocell beams, at the floor level of the box, creating photocell detection (Kungsbacka mät-och reglerteknik AB, Fjärås, Sweden) allowed a computer-based system to register the activity of the mice. The locomotor activity was defined as the accumulated number of new photocell beams interrupted during a 30 min period. Neither water nor food was available to the animal during the locomotor activity experiment.
Locomotor activity testing procedure
On the day of the experiment the guide cannula was used to facilitate drug administration into the VTA. Before initiating the experiment a dummy cannula was carefully inserted and retreated into the guide cannula to remove clotted blood and to hamper spreading depression. Spreading depression occurs as a result of injuries to brain tissues, causing release of ions and other compounds, and thereby interfering with the animal's true behaviour. The mice were left to habituate individually in the locomotor box for 1 h before drug challenge and initialization of the experiment. Next, αCtxMII (5 nmol/1 μl), the αCtxPIA-analogue (5, 10 and 20 nmol/1 μl), or Ringer solution was carefully administered bilaterally into the VTA using a cannnula connected to a 5 μl syringe (Kloehn, microsyringe; Skandinaviska Genetec AB, V. Frölunda, Sweden). The drug was administered over 1 min; the cannula was left in place for another minute and was then retracted and inserted to the contra-lateral guide cannula and the drug administration procedure was repeated. The mice were returned to the locomotor activity boxes after drug administration. Twenty minutes later ethanol (1.75 g/kg) or vehicle [0.9% sodium chloride solution (saline); 12 ml/kg] was administered intraperitoneally (i.p.). The mice in each group received drug treatment only once. To avoid any influence of injection-induced hypermotility the measurement of locomotor activity started 5 min after the ethanol/vehicle injection and was subsequently measured for 30 min and the accumulated counts during this period were then compared between groups. In the locomotor activity experiments with αCtxMII 6–8 mice were included in each group. In the experiments with αCtxPIA-analogue 6–10 observations were included in each group, except from Ringer–NaCl (n = 17) and Ringer–EtOH (n = 20) due to pooling from two experiments. Neither food nor water was available to the mice during the locomotor activity experiment.
Microdialysis procedure
Surgical procedure
The mice were implanted with both a microdialysis probe (Waters et al., 1993) aiming at the N.Acc. for measuring extracellular DA levels and an ipsilateral guide cannula aiming at the VTA for local drug administration. The location of the probe and the ipsilateral guide cannula was alternated to both the left and right side of the brain. The coordinates for N.Acc. relative to the bregma were anterior +1.5 mm, lateral to midline ±0.8, and ventral −4.7 mm and the coordinates for VTA relative to the bregma were posterior −3.4 mm, lateral to midline ±0.5 mm, and ventral −1.0 mm from the brain surface (Franklin and Paxinos, 1996). At time of the experiment the cannula was extended another 3.8 mm ventrally beyond the tip of the guide cannula for drug administration.
The surgical procedure was performed as described above, where first the probe was slowly lowered into position and anchored to the screw in the skull bone with dental cement (Dentalon Plus; Angthós AB). Second the guide cannula was lowered into position and was anchored to the probe with dental cement (Dentalon Plus; Angthós AB). The mice were housed individually in the same type of plastic cages (Macrolon III) as before the operation with food and water supplies ad libitum. The animals were allowed to recover for 4 days before the microdialysis experiment. The exposed tip of the dialysis membrane (20 000 kDa cut off with an o.d./i.d. of 310/220 μm, HOSPAL, Gambro, Lund, Sweden) of the probe was 1 mm.
Microdialysis drug treatment paradigm
The microdialysis technique enables concentration measurements of neurotransmitters in awake, freely moving animals. In these experiments the effects of the αCtxPIA-analogue on ethanol-induced DA-overflow in the N.Acc. were investigated.
On the day of the experiment immediately before start of the experiment, a dummy cannula was carefully inserted and retracted into the guide cannula to remove clotted blood and to hamper spreading depression. Next, the probe was connected to a microperfusion pump (U-864 Syringe Pump; AgnThós AB) and perfused with Ringer solution at a rate of 1.5 μl/min. After 1 h of habituation to the microdialysis perfusion set-up, perfusion samples were collected every 20 min. After collection of five samples, ethanol (1.75 g/kg, i.p.) was administered. Three hours later the αCtxPIA-analogue (5 nmol/1 μl) or Ringer solution was administered locally into the VTA during 1 min; the cannula was left in place for another minute and was then rejected. Twenty minutes after the local drug administration an additional ethanol (1.75 g/kg, i.p.) or vehicle (saline: 12 ml/kg, i.p.) injection followed, and another four samples were collected. A total of 7–10 mice were included in each group. Neither water nor food was available to the animal during the microdialysis experiment.
Biochemical assay
The DA levels in the dialysates were determined by means of HPLC with electrochemical detection (HPLC-EC). A pump (Gyncotec P580A; Kovalent AB, V. Frölunda, Sweden), an ion exchange column (2.0 × 100 mm, Prodigy 3 μm, Skandinaviska GeneTec AB, Kungsbacka, Sweden), and a detector (Antec Decade; Antec Leyden, Zoeterwoude, The Netherlands) equipped with a VT-03 flow cell (Antec Leyden) were used. The mobile phase (pH 5.6) consisting of sulfonic acid 10 mM, citric acid 200 mM, sodium citrate 200 mM, 10% EDTA, and 30% MeOH, which was vacuum filtered by using a 0.2 μm membrane filter (GH Polypro; PALL Gelman Laboratory, Lund, Sweden), was used. The mobile phase was delivered at a flow rate of 0.2 ml/min passing a degasser (Kovalent AB), and the analyte was oxidized at +0.4 V.
Verification of probe and/or guide cannula placement
After the experiments were completed, the location of the probe, cannula, or both was verified. The mice were decapitated, probes were perfused with pontamine sky blue 6BX to facilitate probe identification, and the brains were mounted on a vibroslice device (752M Vibroslice; Campden Instruments Ltd, Loughborough, UK). The brains were cut in 50 μm sections and the location of the probe or cannula was determined by gross observation using light microscopy. Only mice with probe placement in the N.Acc. and/or guide cannula placement in the VTA were included in the statistical analysis (Fig. 2).
Drugs
αCtxMII and the αCtxPIA-analogue were synthesized (cf. Peptide synthesis) and at the experiment the drugs were dissolved in Ringer solution: 140 mM NaCl, 1.2 mM CaCl2, 3.0 mM KCl, and 1.0 mM MgCl2. The dose of αCtxMII (5 nmol/1 μl) used was base on dose-finding experiments to select the highest dose that did not affect locomotor activity per se (data not shown), and doses of 5, 10, and 20 nmol/1 μl were used for the αCtxPIA-analogue during the locomotor activity testing. The dose of 5 nmol/1 μl for the αCtxPIA-analogue was used, since the higher doses (10 and 20 nmol) did not affected locomotor activity per se and therefore to be co-adherent with previous experiments with αCtxMII.
Administration of αCtxMII, the αCtxPIA-analogue, or Ringer solution was performed locally into the VTA at a volume of 1 μl ethanol (VWR International AB, Stockholm, Sweden) was diluted in a 0.9% sodium chloride solution to a 15% (weight/volume) and was administrated i.p.
Statistical analyses
The data from the locomotor activity studies were evaluated by one- or two-way analysis of variance (ANOVA) followed by the Fisher protected least significant difference test (PLSD) for comparisons between treatments.
The microdialysis data were analysed by paired or unpaired t-tests. The baseline DA concentration preceding the first ethanol-induced increase in DA-overflow was defined as the averaged concentration of the two consecutive samples obtained before ethanol challenge (time point 0 min) and the peak increase was defined as the averaged concentration of the two consecutive samples obtained at 80 and 100 min. The baseline DA concentration preceding the second ethanol-induced increase in DA-overflow was defined as the averaged concentration of the two consecutive samples obtained at 160 and 180 min and the peak increase was defined as the averaged concentration of the two consecutive samples obtained at 220 and 240 min. Paired t-tests were used to investigate the maximum DA increase for the respective peaks in each treatment group. Differences between treatment groups in ethanol-induced DA-overflow were analysed by unpaired t-test. A P-value < 0.05 was considered as statistically significant and a P-value greater than 0.05 was considered not significant (n.s.). Error bars in the figures represent standard error of the mean (SEM).
Verification of probe and or cannula(s) placement
Only mice with correct cannula placement into the VTA and/or probe placement within the N.Acc. were included in the statistical analysis. The location of the microdialysis probes within the N.Acc. (Fig. 2) and the ventral tegmental guide cannula (Fig. 2) were verified by gross observation, using light microscopy.
RESULTS
Peptide synthesis
The minor modifications to the already existing synthesis protocol (Cartier et al., 1996) were successful. Pure products, i.e. the correctly cyclized peptides αCtxMII and the αCtxPIA-analogue, were obtained in satisfactory yields (>95 and >90%, respectively). The purity and identity of the peptides were confirmed by HPLC and FAB-MS analyses, respectively. In addition, co-injection of synthesized and purchased αCtxMII on analytical HPLC showed an identical elution profile.
Locomotor activity experiments with synthesized α-conotoxin mii
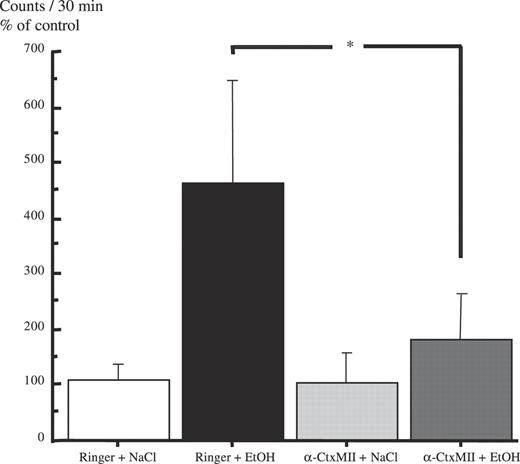
The results of the effect of synthesized αCtxMII (5 nmol/1 μl) on ethanol-induced locomotor stimulation in mice are presented in Fig. 3. A statistically significant overall effect of treatment was observed [F(3,24) = 3.067, P = 0.0472, one-way ANOVA]. Ethanol (1.75 g/kg, i.p.) caused a marked and statistically significant locomotor stimulation in mice in comparison with vehicle-treated animals (P < 0.05, Fisher PLSD). αCtxMII (5 nmol/1 μl, bilaterally administered into the VTA, −20 min) had no effect per se on locomotor activity (n.s. compared with Ringer solution–vehicle, Fisher PLSD), but statistically significantly reduced the ethanol-induced locomotor stimulation (P < 0.05 compared with Ringer solution–Ethanol, Fisher PLSD) [the locomotor activity did not differ from vehicle-treated animals (n.s., Fisher PLSD)].
Local administration of synthesized α-conotoxin MII into the VTA antagonizes the ethanol-induced locomotor stimulation. Effects of synthesized α-conotoxin MII (5 nmol), bilaterally and locally administered into the VTA, on ethanol-induced (EtOH, 1.75 g/kg, i.p.) locomotor stimulation in mice. Shown are the mean values ± SEM of 6–8 observations in each group. (*P < 0.05, compared with Ringer+NaCl, if not otherwise is indicated, *P < 0.05, comparing Ringer+EtOH with α-CtxMII+EtOH, Fisher PLSD test, after significant analysis of variance). Ringer = Ringer solution.
Locomotor activity experiments with the synthesized α-conotoxin PIA-analogue
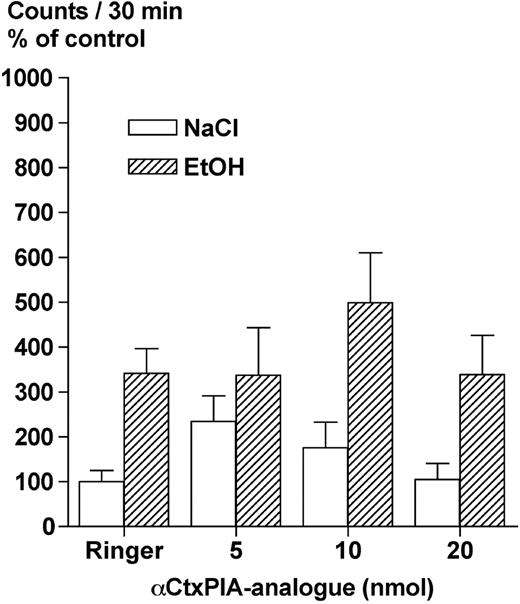
The results of the effect of the synthesized αCtxPIA-analogue (5, 10, 20 nmol/1 μl) on ethanol-induced locomotor stimulation in mice are presented in Fig. 4. A statistically significant overall effect of treatment was observed [F(1,79) = 19.9, P < 0.001, two-way ANOVA]. Collapsed over all doses of the αCtxPIA-analogue used, ethanol (1.75 g/kg, i.p.) caused a statistically significant locomotor stimulation in the mice in comparison with saline-treated animals (P < 0.001, Fisher PLSD). The αCtxPIA-analogue (5, 10, and 20 nmol/1 μl, bilaterally administered into the VTA; −20 min) in the doses tested had no statistically significant effect per se on locomotor activity [F(3,37) = 2.37, n.s., one-way ANOVA, effect of treatment]. Neither did any of the doses reduce the ethanol-induced locomotor stimulation in the mice (n.s., Fisher PLSD).
Local administration of the synthesized α-conotoxin PIA-analogue into the VTA does not antagonize the ethanol-induced locomotor stimulation. Effects of the synthesized α-conotoxin PIA-analogue (5, 10 and 20 nmol), bilaterally and locally administered into the VTA, on ethanol-induced (EtOH, 1.75 g/kg, i.p.) locomotor stimulation in mice. Shown are the mean values ± SEM of 6–10 observations in each group, except from Ringer–NaCl (n = 17) and Ringer–EtOH (n = 20) due to pooling from two experiments. Ringer = Ringer solution.
Microdialysis experiments with synthesized α-conotoxin pia
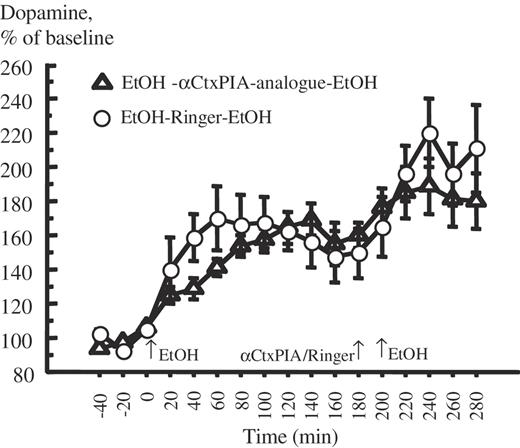
The results of the effect of the αCtxPIA-analogue (5 nmol/1 μl) or Ringer on ethanol-induced DA-overflow in N.Acc. in awake, freely moving mice are presented in Fig. 5. Statistical analysis of the data showed that the first ethanol administration (1.75 g/kg i.p.) statistically significantly increased the extracellular DA levels in N.Acc. in both treatment groups in comparison with the preceding baseline concentrations. The maximal DA levels in N.Acc. were observed ∼90 min after the ethanol administration. EtOH–Ringer–EtOH (+67.9%; P < 0.01, paired t-test), EtOH–αCtxPIA-analogue–EtOH (+53.7%; P < 0.01, paired t-test). These peaks were not significantly different in amplitude (unpaired t-test).
Local administration of the synthesized α-conotoxin PIA-analogue into the VTA does not antagonizes the ethanol-induced accumbal dopamine-overflow. Effect of the synthesized α-conotoxin PIA-analogue (5 nmol), unilaterally and locally administered into the VTA, on ethanol-induced (EtOH, 1.75 g/kg, i.p.) dopamine-overflow in nucleus accumbens in mice measured by in vivo microdialysis. Ethanol was administered the first time at 0 min; Ringer or α-conotoxin PIA was administered at 180 min. After 20 min from the beginning of drug administration ethanol was administered the second time (EtOH, 1.75 g/kg, i.p.). Shown are mean values ± SEM of 7–10 observations in each group. Ringer = Ringer solution.
A second administration of ethanol (1.75 g/kg, i.p.), after administration of Ringer solution (1 μl, unilaterally into the VTA; −20 min) or the αCtxPIA-analogue, also caused a statistically significant increase in accumbal DA levels (P < 0.05, compared with the preceding baseline in both treatment groups). EtOH–Ringer–EtOH (+59.9 ± 8.4%; P < 0.001, paired t-test), EtOH–αCtxPIA-analogue–EtOH +29.2 ± 10.7%; P < 0.05, paired t-test). Neither these peaks were significantly different in amplitude (P > 0.05, unpaired t-test).
In a separate control experiment, the administration schedule EtOH–Ringer–Saline was used. Also in this experiment ethanol produced an increase in DA-overflow but Ringer/Saline did not cause any significant effects on DA-overflow. In a second control experiment, the administration schedule Saline–αCtxPIA-analogue–Saline was used. None of these treatments caused a statistically significantly change in accumbal DA levels (P > 0.05, paired t-tests, data not shown). These results show that neither the cannula insertion, i.p. injection, volume of ringer infused, nor αCtxPIA-analogue, per se, had any significant effects on ethanol-induced DA-overflow in N.Acc..
DISCUSSION
In previous publications we have shown that the
It cannot be excluded that the synthesized αCtxPIA-analogue is different from αCtxPIA. However, this appears less likely since the nAChR subunit selectivity of 4/7 α-conotoxins has been reported to involve the amino acid residues in the central and the C-terminal part of the peptide, whereas the N-terminal appears to be of less importance (McIntosh et al., 1999; Arias and Blanton, 2000; Dutertre and Lewis, 2004; Everhart et al., 2004; Dutertre et al., 2005). Specifically, the bulky charged N-terminal protrusion of αCtxPIA has been suggested to be of less importance for its interaction with the α6 subunit of the nAChR (Chi et al., 2005). In the present series of experiments an analogue to αCtxPIA, the αCtxPIA-analogue, was used in which the N-terminal arginine had been removed. To maintain the selectivity to the
We show that the modified synthetic method allowed efficient production of αCtxMII (Cartier et al., 1996) and the αCtxPIA-analogue. Results from analytical HPLC and FAB-MS analysis confirmed the purity and the identity, respectively, of the synthesized peptides.
First we found that local administration of synthesized αCtxMII antagonized ethanol-induced locomotor stimulation, which besides confirming our previous results with the commercially available αCtxMII (Larsson et al., 2004) indicates that the αCtxMII sensitive receptors (i.e. the
It should be considered that, in addition to nAChRs, muscarinic acetylcholine receptors have been shown to be present in the VTA (Yeomans and Baptista, 1997; Yeomans et al., 2001; Forster et al., 2002; Miller et al., 2005). Interestingly, muscarinic acetylcholine receptors have been demonstrated to play a role in natural reward (Yeomans and Baptista, 1997; Rada et al., 2000) and could consequently have an additional role in mediating the stimulatory and DA-enhancing effects of ethanol. It should be emphasized that the rewarding profile of ethanol is orchestrated by several other neurohumoral systems including glutamate, 5-HT, GABA, and opioid peptides (Engel et al., 1992; Koob, 1992). Thus, the possibility that the effects of αCtxMII could be due to non-nicotinic receptors cannot be excluded.
From this series of experiments it cannot be determined whether the stimulatory, rewarding, and DA-enhancing effects of ethanol mediated via nAChR are exerted via a direct interaction of ethanol with nAChR in the VTA and/or indirectly via increased extracellular ACh in the VTA. Proof of concept for the latter hypothesis show a concomitant release of ACh in the VTA and DA in the N.Acc. upon voluntary ethanol intake (Larsson et al., 2005), thus implicating that a cholinergic input to the VTA mediates the rewarding effects of ethanol. The cholinergic excitatory input to the VTA, foremost originating in the pedunculopontine tegmental nucleus and laterodorsal tegmental nucleus (Woolf, 1991; Butcher and Woolf, 2003), has been suggested to be an important part of neural circuits mediating both natural as well as drug-induced reward (Lanca et al., 2000; Rada et al., 2000). However, it cannot be excluded that ethanol, e.g. as a co-agonist to ACh on nAChR (Marszalec et al., 1999), interacts directly with nAChR on neurons, e.g. dopaminergic cellbodies, in the VTA.
In conclusion, the present data suggest that the stimulatory and DA-enhancing effects of ethanol might be mediated via αCtxMII- rather than αCtxPIA-analogue-sensitive receptors in the VTA, suggesting an important mediating role for the
The authors are grateful for the excellent help from Mrs Gun Andersson and Mr Kenn Johannessen. This work was supported by grants from the Swedish Research Council (no. 4247 and no. 621-2004-4571) and NIDA 2 R01 10765-04A1, the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly, The Swedish Labour Market Insurance (AFA), Wilhelm and Martina Lundgrens Scientific Foundation, Rådman and Fru Ernst Collianders Foundation, the Tornspiran Foundation, the Lundbeck Foundation, Knut and Alice Wallenberg Foundation (no. 98.176), The Adlerbertska Foundation, the Filip Lundbergs Foundation, The Längman Cultural Foundation.
REFERENCES
Arias, H. R. and Blanton, M. P. (
Ayers, J., Ruff, C. F. and Templer, D. I. (
Batel, P., Pessione, F., Maitre, C. et al. (
Bien, T. H. and Burge, R. (
Blomqvist, O., Ericson, M., Engel, J. A. et al. (
Butcher, L. L. and Woolf, N. J. (
Carmody, T. P., Brischetto, C. S., Matarazzo, J. D. et al. (
Cartier, G. E., Yoshikami, D., Gray, W. R. et al. (
Champtiaux, N., Han, Z. Y., Bessis, A. et al. (
Charpantier, E., Barneoud, P., Moser, P. et al. (
Chi, S.-W., Lee, S.-H., Kim, D.-H. et al. (
Clarke, P. B., Fu, D. S., Jakubovic, A. et al. (
Clarke, P. B. and Pert, A. (
Cui, C., Booker, T. K., Allen, R. S. et al. (
Di Chiara, G. (
DiFranza, J. R.and Guerrera, M. P. (
Dowell, C., Olivera, B. M., Garrett, J. E. et al. (
Dutertre, S. and Lewis, R. J. (
Dutertre, S., Nicke, A. and Lewis, R. J. (
Engel, J. A., Fahlke, C., Hard, E. et al. (
Ericson, M., Molander, A., Löf, E. et al. (
Everhart, D., Cartier, G. E., Malhotra, A. et al. (
Forman, S. A., Righi, D. L. and Miller, K. V. (
Forman, S. A. and Zhou, Q. (
Forster, G. L., Yeomans, J. S., Takeuchi, J. et al. (
Franklin, K. B. J. and Paxinos, G. (
Grant, B. F. (
Istvan, J. and Matarazzo, J. D. (
Klink, R., de Kerchove d'Exaerde, A., Zoli, M. et al. (
Koob, G. F. (
Lanca, A. J., Adamson, K. L., Coen, K. M. et al. (
Larsson, A., Svensson, L., Söderpalm, B. et al. (
Larsson, A. and Engel, J. A. (
Larsson, A., Jerlhag, E., Svensson, L. et al. (
Larsson, A., Edström, L., Svensson, L. et al. (
Le Novere, N., Corringer, P. J. and Changeux, J. P. (
Lê, A. D., Corrigall, W. A., Harding, J. W. et al. (
Maisonneuve, I. M. and Glick, S. D. (
Marszalec, W., Aistrup, G. L. and Narahashi, T. (
McIntosh, J. M., Santos, A. D. and Olivera, B. M. (
Miller, N. S. and Gold, M. S. (
Miller, A. D., Forster, G. L., Yeomans, J. S. et al. (
Nicke, A., Wonnacott, S. and Lewis, R.J. (
Rada, P. V., Mark, G. P., Yeomans, J. J. et al. (
Rose, J. E., Brauer, L. H., Behm, F. M. et al. (
Vailati, S., Hanke, W., Bejan, A. et al. (
Walton, R. G. (
Waters, N., Lagerkvist, S., Lofberg, L. et al. (
Wonnacott, S., Drasdo, A., Sanderson, E. et al. (
Woolf, N. J. (
Wu, G., Tonner, P. H. and Miller, K. W. (
Yeomans, J. and Baptista, M. (
Yeomans, J., Forster, G. and Blaha, C. (
Author notes
1Institute of Physiology and Pharmacology, Department of Pharmacology, Göteborg University, Box 431, SE 405 30 Göteborg, Sweden
2Department of Chemistry, Medicinal Chemistry, Göteborg University, SE 412 96 Göteborg, Sweden