-
PDF
- Split View
-
Views
-
Cite
Cite
YOICHIRO HOSHINO, NAHO MURATA, KOICHI SHINODA, Isolation of Individual Egg Cells and Zygotes in Alstroemeria Followed by Manual Selection with a Microcapillary-connected Micropump, Annals of Botany, Volume 97, Issue 6, June 2006, Pages 1139–1144, https://doi.org/10.1093/aob/mcl072
Close - Share Icon Share
Abstract
• Aims To develop a procedure for isolating living egg cells and zygotes from Alstroemeria ovules.
• Scope An attempt was made to isolate egg cells and zygotes from the ovules of Alstroemeria aurea. The ovules were histologically observed using a clearing procedure which revealed the localization and sizes of the embryo sacs and egg apparatus within the ovules. For the isolation of egg cells, ovules were cut into sections with a surgical blade and treated with an enzyme solution. Subsequently, these ovule sections were dissected using a glass needle under an inverted microscope. Egg cells successfully isolated by this procedure were collected using microcapillaries connected to a micropump. For zygote isolation, ovules were excised from ovaries 24 h after self-pollination. By treating excised ovules with an enzyme solution and subsequently dissecting them using a glass needle, zygotes were successfully isolated from the ovules and collected with a microcapillary. The isolated zygotes were associated with pollen tubes and one of the synergids. Egg cells and zygotes were viable for up to 2 h following isolation, as determined by fluorescein diacetate staining.
• Conclusions The procedures for isolating egg cells and zygotes in Alstroemeria were established, and each egg cell and zygote was captured with a microcapillary.
INTRODUCTION
Recently, techniques have been developed for the isolation and culture of female gametes (reviewed in Theunis et al., 1991) and also for plant regeneration from the in-vitro-fertilized egg cells of Zea mays (Kranz and Lörz, 1993; Kranz, 1999; Kranz et al., 2004) and from the zygote protoplasts of Hordeum vulgare (Holm et al., 1994), Z. mays (Leduc et al., 1996), Triticum aestivum (Kumlehn et al., 1998) and Oryza sativa (Zhang et al., 1999). The isolated gametes are expected to find various innovative applications such as the direct observation of fertilization processes in vitro, studies of the mechanisms of recognition, adhesion and fusion of gametes and in vitro fertilization studies for breeding by crossing distantly related species. However, in most angiosperms, it is still difficult to manipulate female gametophytes because their development generally occurs deep within the tissues of sporophytic ovules. To date, enzymatic procedures for the isolation of female gametes or embryo sacs have been described for several plant species, including Torenia fournieri (Mòl, 1986), Lilium longiflorum (Wagner et al., 1989a), Zea mays (Wagner et al., 1989b), Petunia (Van Went and Kwee, 1990), Crinum asiaticum (Ohshika and Ikeda, 1994), Brassica napus (Katoh et al., 1997), Dianthus species (Hoshino et al., 2000) and Helianthus annuus (Popielarska and Przywara, 2003).
The genus Alstroemeria belongs to the family Alstroemeriaceae and includes more than 60 species, many of which are ornamentals. The Alstroemeria originated in South America and through interspecific hybridization and mutation breeding numerous cultivars, which are currently used as cut flowers and potted plants, have been propagated throughout the world. Novel regeneration systems established in Alstroemeria from isolated egg cells, in-vitro-fertilized egg cells, and zygotes can be expected to be used for further breeding of the species of this genus through direct gene transfer and in-vitro fertilization between sexually incompatible species. In-vitro fertilization is considered to be a useful approach for the generation of interspecific hybrids within this genus. To facilitate future study of in-vitro fertilization, procedures were developed for the isolation of egg cells and zygotes produced in vivo in Alstroemeria aurea, using enzymatic treatments and microdissection with glass needles.
MATERIALS AND METHODS
Plant materials and the collection of flowers
The flowers of A. aurea were collected from plants grown in a greenhouse. Alstroemeria flowers are dichogamous and protandrous. In the 5–7 d following anthesis, there is a staggered dehiscence of the six anthers: approximately one anther releases pollen per day. The styles elongate following dehiscence of all the anthers. In order to isolate egg cells, flowers were harvested 1–3 d after anthesis, but before pollination. Zygotes were isolated from flowers following self-pollination. These flowers were used both for histological examination of the ovules and for the isolation of egg cells or zygotes.
Observations of embryo sacs in whole-mount ovules
Microscopic observations using the clearing procedure described in Hoshino et al. (2000) were undertaken to determine the location of the embryo sac, including the egg cell, in whole ovule mounts. After removal of the perianths from harvested flowers, ovaries were fixed in FAA solution (formalin : acetic acid : 50 % ethanol, 5 : 5 : 90, v/v/v) for 1–3 d (Sass, 1958). Whole ovules and embryo sacs were prepared for histological examination by washing the fixed ovaries with distilled water and staining with modified Mayer's acid haemalaum (Lillie, 1965). After staining for 1 h at room temperature, the ovules were partially destained in distilled water for 1–3 h, depending on individual staining intensity. Subsequently, the ovules were successively dehydrated in a series of ethanol solutions (50 % for 2 h, 75 % for 2 h and 95 % for 24 h), then cleared by successive transfers to 95 % ethanol : benzyl benzoate (2 : 1, v/v/v), 95 % ethanol : benzyl benzoate (1 : 2, v/v/v) and benzyl benzoate : dibutyl phthalate (1 : 1, v/v/v) at intervals of >1 h according to the method of Crane and Carman (1987) with several modifications. In the present study, an FAA solution was used instead of Carnoy's fixative and staining with modified Mayer's acid haemalaum was added to the protocol of Crane and Carman (1987). Treated ovules were mounted in benzyl benzoate : dibutyl phthalate in the well (4 mm depth) of a cavity glass slide and covered by a coverslip. Observations were made using an inverted microscope (IX-70, Olympus) incorporating Nomarski differential interference equipment.
Isolation of the egg cells by enzymatic treatment and microdissection
After removal of perianths from harvested flowers, ovaries were surface-sterilized with a sodium hypochlorite solution (1 % active chlorine) for 10 min and rinsed three times with sterilized distilled water. The ovaries were cut longitudinally into three sections using a surgical blade. The ovary walls were then peeled off, and the ovules detached from the placenta. The efficacy of three excision procedures was then examined with regard to the following: (a) removal of the micropylar region; (b) removal of the chalazal region; and (c) excision of the funicle from each ovule with a surgical blade under a dissecting microscope. The ovule sections were placed into 2 mL of filter-sterilized (Millipore; 0·45-µm pore size) enzyme solution in a plastic Petri dish (35 mm × 15 mm). The enzyme solution used comprised 2 % Cellulase Onozuka RS (Yakult Pharmaceutical Co. Ltd, Japan), 0·5 % Macerozyme R-10, 0·05 % Pectolyase Y-23 (Seishin Pharmaceutical Co. Ltd, Japan), 10 mm CaCl2·2H2O, 5 mm 2-(N-morpholino)-ethanesulfonic acid and 0·5 m mannitol. The pH of the solution was adjusted to 5·8 before filter sterilization. The ovule sections were subsequently incubated at 25 °C for 2–3 h. After incubation, in order to isolate egg cells, ovules were dissected with hand-made glass needles under an inverted microscope (Axiovert 200; Carl Zeiss, Oberkochen, Germany). Isolated egg cells were collected with a microcapillary connected to a micropump (Nano Spuit; IKEDA Scientific Co. Ltd, Japan) and transferred to a 0·5 m (555 mosmol kg–1 H2O) mannitol solution. The design of the Nano Spuit was based on the reports of Koop and Schweiger (1985) and Kranz et al. (1991). The microcapillaries were connected via fine-bore tubes to a computer-controlled micropump, and this hydraulic system has both dispenser and suction functions.
Zygote isolation
In order to isolate zygotes produced in vivo, a time course of pollen germination to pollen tube entry into the micropylar region was monitored by aniline blue staining of pistils following self-pollination. Pistils were harvested at 5 min, 10 min, 30 min, 1 h, 3 h, 6 h, 12 h, 18 h, 24 h or 30 h after self-pollination. The ovaries were then fixed in Farmer's solution (acetic acid : ethanol, 1 : 3, v/v/v) at room temperature for 24 h, and retained in 70 % ethanol at 4 °C. For observations of pollen tubes, fixed pistils were hydrolysed in 1 n NaOH for 15 min at 60 °C and then washed three times for 10 min with distilled water. Pistils were stained overnight with a 0·1 % solution of aniline blue in 0·1 n K3PO4. Stained whole pistils were dissected longitudinally, placed onto a slide glass and squashed in 50 % glycerol diluted by distilled water. Samples were observed using an epifluorescence microscope (Axiovert 200; Zeiss, Oberkochen, Germany). The fluorescence from the pollen tubes was detected with the excitation filter set to 1. Images were taken with a camera (DS-L1; Nikon, Tokyo, Japan).
Isolation of zygotes produced in vivo from ovules after pollination was attempted using the same procedure as that described for egg isolation.
Viability test
The viability of isolated egg cells and zygotes was assessed with fluorescein diacetate (FDA; Sigma-Aldrich) staining (Heslop-Harrison and Heslop-Harrison, 1970). For the preparation of a stock solution, 15 mg FDA was dissolved in 5 ml acetone. A working solution of 5 µg mL–1 was prepared by diluting the stock solution with 0·5 m mannitol. Individual egg cells and zygotes were stained with the FDA working solution for 5 min and then observed under the epifluorescence microscope. The fluorescence was detected with the filter set to 17. Images were captured with the DS-L1 camera.
RESULTS AND DISCUSSION
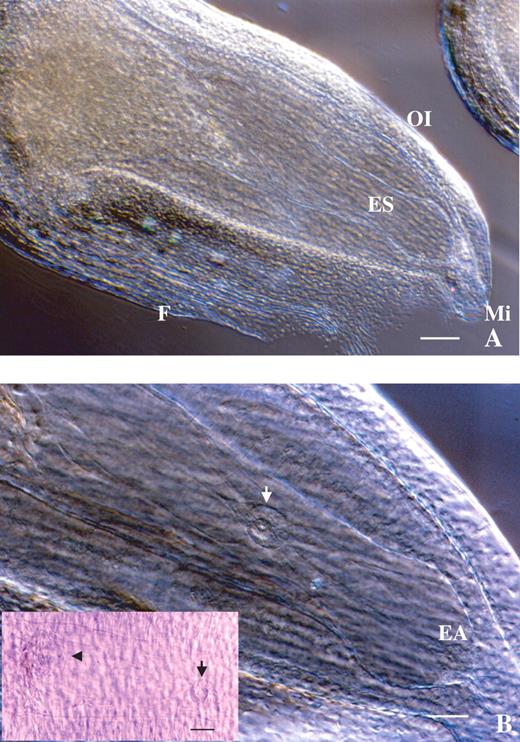
Observations using a microscopic showed that Alstroemeria has an anatropous-type ovule (Fig. 1A). The embryo sac, surrounded by nucellar tissue with an outer and inner integument, was found to be very large – approx. 890 µm in longitudinal diameter (Fig. 1). Thick nucellus tissue could be observed at the micropylar region. Figure 1B shows that the egg apparatus and central cell are visible in the embryo sac. The central cell has one very large nucleus (approx. 45 µm in diameter) which originates from the fusion of the two polar nuclei. The antipodal cells are located on the chalazal side (Fig. 1B).
Microscopic observation of an Alstroemeria ovule using the clearing procedure. (A) Whole ovule showing the large embryo sac within. ES, Embryo sac; F, funicle; OI, outer integument; Mi, micropyle. Scale bar = 120 μm. (B) Magnification of the inside of an ovule. The egg apparatus (EA) and fused polar nuclei (white and black arrows) are in focus. The antipodal cells (arrowhead) are also observed in the left of the frame. Scale bar = 60 μm.
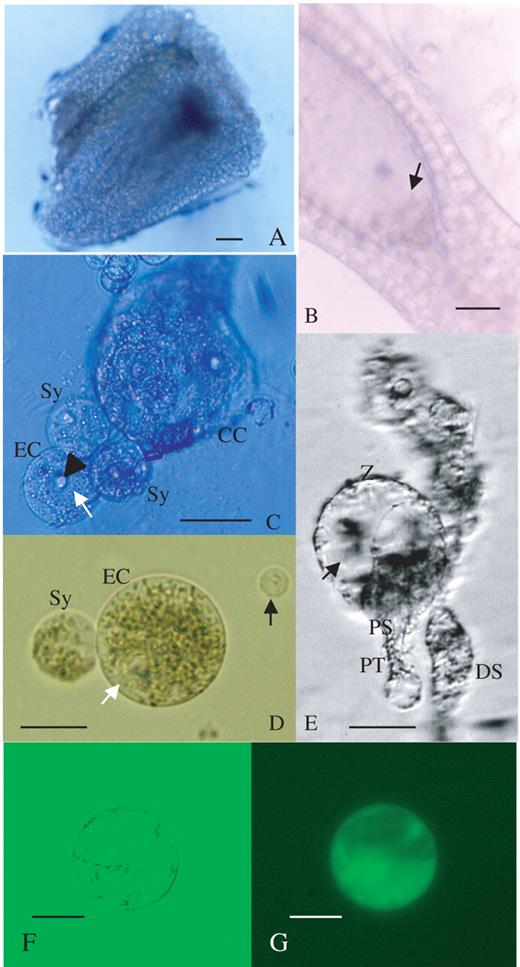
Based on the histological observations of the ovule and embryo sac, an attempt was made to isolate the female gamete (egg cell) by enzyme maceration in combination with microdissection with a glass needle. When whole ovules were treated with enzyme solutions for 2–3 h, the ovules could not be dissected with glass needles under an inverted microscope since the outer integument was not digested by commercial enzymes. In view of this, several procedures for excising a part of the ovule were tested in order to enhance enzyme solution permeability and thus render ovule tissues more amenable to dissection. Ovules were detached from the placenta and the following three excision procedures were examined: (a) removal of the micropylar region; (b) removal of the chalazal region; and (c) excision of the funicle from each ovule with a surgical blade under a dissecting microscope. A comparison of these techniques revealed that removal of the chalazal region (Fig. 2A) was effective in promoting the digestion of sporophytic cells surrounding the embryo sac. Furthermore, this treatment facilitated the dissection of the ovule and, eventually, egg cell isolation. Sporophytic protoplasts could be obtained from excised ovules after enzyme treatment for 30 min. After incubation for 2–3 h, the integuments were removed in the enzyme solution with glass needles (Fig. 2B). Finally, it was possible to isolate egg cells from ovules in which both the funicle and parts of nucellus tissues on the chalazal side had been excised, by carefully dissecting enzyme-treated ovules from the chalazal side with delicate manipulation of glass needles under an inverted microscope (Fig. 3). The isolated egg cells were spherical and vacuolated (Fig. 2C, D). Occasionally, synergids and the central cell were isolated together with an egg cell (Fig. 2C). Isolation efficiency was approximately two to five cells per hour. The main features of the isolated egg cells are summarized as follows.
Large vacuoles were observed in the egg cells (Fig. 2C).
The vacuoles were located at the periphery of the egg cells (Fig. 2D).
No chloroplasts or starch grains were observed.
The nucleus of the egg cell was relatively large compared with that of other somatic cells released from the ovules (Fig. 2D).
The average diameters of egg cells and synergids were 50·4 and 45·2 µm, respectively. The average values were based on the measurement of ten egg cells and synergids using an ocular micrometer under an inverted microscope.
Isolation processes of egg cell and zygote from an Alstroemeria ovule, and viability test for the egg cell by FDA staining. (A) An ovule section during enzyme treatment. Scale bar = 60 μm. (B) Dissection of ovule with glass needles. Integuments were peeled off and the egg apparatus was exposed (arrow). Scale bar = 60 μm. (C) Immediately after isolation of the egg cell (EC), with synergids (Sy) and a fragment of the central cell (CC). Vacuoles (white arrow) were observed in the egg cell. The nucleus of the egg cell (arrowhead) was conspicuous. Scale bar = 60 μm. (D) An isolated egg cell (EC) with one synergid (Sy) was transferred to mannitol solution by a microcapillary. The white arrow indicates the position of vacuoles. A sporophytic cell (black arrow) is shown for comparison. Scale bar = 30 μm. (E) An isolated zygote (Z) accompanied by a persisting synergid (PS) and pollen tube (PT). A degenerated synergid (DS) was also observed. The zygote was highly vacuolated. The arrow indicates the vacuoles. Scale bar = 30 μm. (F) An egg cell 2 h after isolation. Scale bar = 30 μm. (G) An epifluorescence image of an isolated egg cell after FDA staining. The egg cell is the same as that in (F). Scale bar = 30 μm.
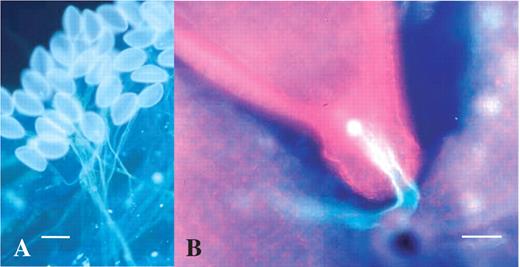
The capture of an egg cell with a mirocapillary connected to a micropump. Serial images (A–C) show the process for absorbing only the egg cell (EC). Scale bar = 100 μm.
In order to examine the culture conditions necessary for zygotes after in vitro fertilization, the isolation of the zygotes was attempted. To measure the interval between pollination and pollen tube discharge, pollen tube elongation was observed by aniline blue staining following self-pollination (Table 1). Observations showed that pollen germination started 1 h after pollination (Table 1 and Fig. 4A). The pollen tubes continued to grow through the style and reached the ovary 18 h after pollination. Pollen tube entry into the micropylar region was observed at 24 and 30 h after pollination (Table 1 and Fig. 4B). The frequencies of pollen tube entry into ovules after 24 h and 30 h were 80 % and 92 %, respectively (Table 1).
Aniline blue staining of pollen tubes on the stigma and in the ovary. (A) Pollen germination on the stigma 6 h after self-pollination. Scale bar = 80 μm. (B) Pollen tube entry into micropyle of the ovule 24 h after self-pollination. Scale bar = 50 μm.
Time course of pollen germination and pollen tube elongation after self-pollination in Alstroemeria
| Time after pollination . | No. of ovaries examined . | Av. no. of pollen germinations on a stigma . | Presence of pollen tube in the pistil . | Av. no. of ovules observed per ovary . | Frequency of pollen tube entry into ovules (%) . |
|---|---|---|---|---|---|
| 5 min | 2 | 0.0 | –* | 21·0 | 0 |
| 10 min | 2 | 0·0 | – | 22·5 | 0 |
| 30 min | 2 | 0·0 | – | 18·0 | 0 |
| 1 h | 2 | 15·0 | Stigma | 17·5 | 0 |
| 3 h | 2 | 118·5 | Stigma | 23·5 | 0 |
| 6 h | 6 | 320·8 | Style | 23·2 | 0 |
| 12 h | 3 | 529·0 | Style | 25·0 | 0 |
| 18 h | 3 | 355·7 | Ovary | 25·3 | 0 |
| 24 h | 5 | 491·8 | Ovary | 19·2 | 80 |
| 30 h | 3 | 475·3 | Ovary | 23·7 | 92 |
| Time after pollination . | No. of ovaries examined . | Av. no. of pollen germinations on a stigma . | Presence of pollen tube in the pistil . | Av. no. of ovules observed per ovary . | Frequency of pollen tube entry into ovules (%) . |
|---|---|---|---|---|---|
| 5 min | 2 | 0.0 | –* | 21·0 | 0 |
| 10 min | 2 | 0·0 | – | 22·5 | 0 |
| 30 min | 2 | 0·0 | – | 18·0 | 0 |
| 1 h | 2 | 15·0 | Stigma | 17·5 | 0 |
| 3 h | 2 | 118·5 | Stigma | 23·5 | 0 |
| 6 h | 6 | 320·8 | Style | 23·2 | 0 |
| 12 h | 3 | 529·0 | Style | 25·0 | 0 |
| 18 h | 3 | 355·7 | Ovary | 25·3 | 0 |
| 24 h | 5 | 491·8 | Ovary | 19·2 | 80 |
| 30 h | 3 | 475·3 | Ovary | 23·7 | 92 |
* No pollen tube was visible.
Time course of pollen germination and pollen tube elongation after self-pollination in Alstroemeria
| Time after pollination . | No. of ovaries examined . | Av. no. of pollen germinations on a stigma . | Presence of pollen tube in the pistil . | Av. no. of ovules observed per ovary . | Frequency of pollen tube entry into ovules (%) . |
|---|---|---|---|---|---|
| 5 min | 2 | 0.0 | –* | 21·0 | 0 |
| 10 min | 2 | 0·0 | – | 22·5 | 0 |
| 30 min | 2 | 0·0 | – | 18·0 | 0 |
| 1 h | 2 | 15·0 | Stigma | 17·5 | 0 |
| 3 h | 2 | 118·5 | Stigma | 23·5 | 0 |
| 6 h | 6 | 320·8 | Style | 23·2 | 0 |
| 12 h | 3 | 529·0 | Style | 25·0 | 0 |
| 18 h | 3 | 355·7 | Ovary | 25·3 | 0 |
| 24 h | 5 | 491·8 | Ovary | 19·2 | 80 |
| 30 h | 3 | 475·3 | Ovary | 23·7 | 92 |
| Time after pollination . | No. of ovaries examined . | Av. no. of pollen germinations on a stigma . | Presence of pollen tube in the pistil . | Av. no. of ovules observed per ovary . | Frequency of pollen tube entry into ovules (%) . |
|---|---|---|---|---|---|
| 5 min | 2 | 0.0 | –* | 21·0 | 0 |
| 10 min | 2 | 0·0 | – | 22·5 | 0 |
| 30 min | 2 | 0·0 | – | 18·0 | 0 |
| 1 h | 2 | 15·0 | Stigma | 17·5 | 0 |
| 3 h | 2 | 118·5 | Stigma | 23·5 | 0 |
| 6 h | 6 | 320·8 | Style | 23·2 | 0 |
| 12 h | 3 | 529·0 | Style | 25·0 | 0 |
| 18 h | 3 | 355·7 | Ovary | 25·3 | 0 |
| 24 h | 5 | 491·8 | Ovary | 19·2 | 80 |
| 30 h | 3 | 475·3 | Ovary | 23·7 | 92 |
* No pollen tube was visible.
Based on these observations of the pollen tubes, ovaries were harvested 24 h after self-pollination for zygote isolation. Zygotes were isolated using the same procedure used for egg cell isolation. The chalazal region was removed from the ovules and ovule sections were treated with the same enzyme solution used for egg cell isolation. After incubation for 2–3 h, zygotes could be isolated by microdissection with glass needles. Zygotes were confirmed by noting the connection between pollen tubes and synergids (Fig. 2E). Isolated zygotes were highly vacuolated (Fig. 2E). Further studies will be necessary to determine the culture conditions needed for plant regeneration from isolated zygotes.
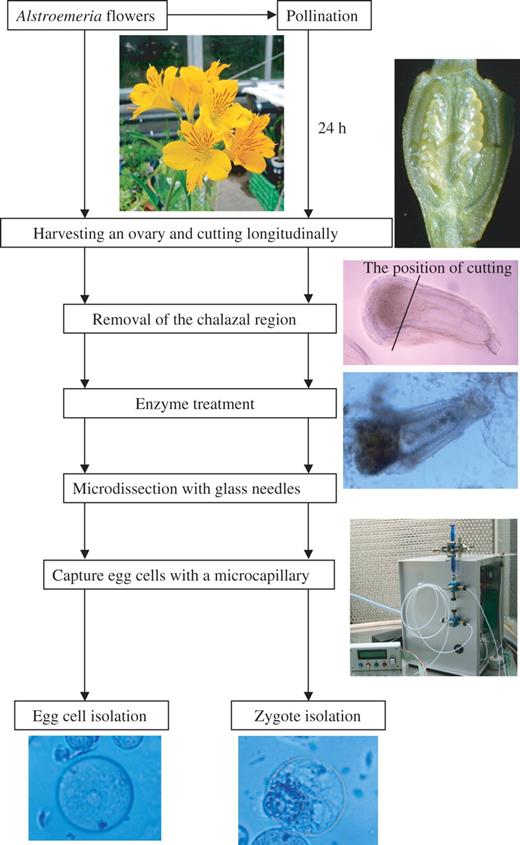
In conclusion, from each ovary containing approx. 30 ovules, approximately two to five egg cells or zygotes were isolated within 1–2 h. Isolated egg cells and zygotes were collected individually in mannitol droplets by using a Nano Spuit, which is a microcapillary-connected micropump. FDA-positive egg cells (Fig. 2F, G) and zygotes were observed 2 h after isolation, and their viability was indicated. The intensity of FDA staining in both egg cells and zygotes became weak after incubation in mannitol for periods >10 h after isolation. To maintain the viability of egg cells and to culture zygotes, a suitable nutritive medium and a nurse cell system should be established. A schematic representation of the procedures for isolating egg cells and zygotes developed in this study is presented in Fig. 5.
Isolated egg cells and zygotes have been utilized in studies on fertilization and early embryogenesis by analysing gene expression (Scholten et al., 2002; Okamoto et al., 2004, 2005; Le et al., 2005). The procedures developed for isolating egg cells and zygotes described in this paper might find further application in studies designed to investigate the regulatory factors controlling fertilization and subsequent embryogenesis. For example, Hoshino et al. (2004) demonstrated the immunocytochemical detection of single cells in microtubules by using isolated egg cells and zygotes and showed fertilization-induced changes in microtubular architecture. These techniques will provide the information necessary to improve the culture conditions for zygotes and early embryos resulting from distant sexual hybridization, by overcoming embryo abortion. At present, the isolation of sperm cells from Alstroemeria pollen grains, which are of a bicellular-type, is being examined. Analyses of mitotic division of the generative nucleus and the formation of sperm cells in pollen tubes are now in progress. The procedures for isolating egg cells and zygotes developed in the present study might offer a new approach to the further study of in vitro fertilization in this species.
We thank Prof. E. Kranz (University of Hamburg, Germany) for his suggestions regarding the development of the microcapillary-connected micropump used in this study. This work was supported in part by a Sasagawa Scientific Research Grant from the Japan Science Society, grants from The Akiyama Foundation and the Inamori Foundation, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
LITERATURE CITED
Crane CF, Carman JG.
Heslop-Harrison J, Heslop-Harrison Y.
Holm PB, Knudsen S, Mouritzen P, Negri D, Olsen FL, Roué C.
Hoshino Y, Nishino E, Mii M.
Hoshino Y, Scholten S, von Wiegen P, Lörz H, Kranz E.
Katoh N, Lörz H, Kranz E.
Koop HU, Schweiger HG.
Kranz E.
Kranz E, Lörz H.
Kranz E, Bautor J, Lörz H.
Kranz E, Hoshino Y, Okamoto T, Scholten S.
Kumlehn J, Lörz H, Kranz E.
Le Q, Gutierrez-Marcos JF, Costa LM, Meyer S, Dickinson HG, Lörz H, et al.
Leduc N, Matthys-Rochon E, Rougier M, Mogensen L, Holm P, Magnard J-L, et al.
Lillie RD.
Mól R.
Ohshika K, Ikeda H.
Okamoto T, Higuchi K, Shinkawa T, Isobe T, Lörz H, Koshiba T, et al.
Okamoto T, Scholten S, Lörz H, Kranz E.
Popielarska M, Przywara L.
Scholten S, Lörz H, Kranz E.
Theunis CH, Pierson ES, Cresti M.
Van Went JL, Kwee HS.
Wagner VT, Kardolus JP, Van Went JL.
Wagner VT, Song YC, Matthys-Rochon E, Dumas C.
Author notes
1Field Science Center for Northern Biosphere, Hokkaido University, Kita 11, Nishi 10, Kita-Ku, Sapporo 060-0811, Japan, 2Division of Innovative Research, Creative Research Initiative ‘Sousei’ (CRIS), Hokkaido University, Kita 21, Nishi 10, Kita-Ku, Sapporo 001-0021, Japan and 3National Agricultural Research Center for Hokkaido Region, Hitsujigaoka 1, Toyohira-Ku, Sapporo 062-8555, Japan