-
PDF
- Split View
-
Views
-
Cite
Cite
Lutz Fromhage, Jutta M. Schneider, Emasculation to plug up females: the significance of pedipalp damage in Nephila fenestrata, Behavioral Ecology, Volume 17, Issue 3, May/June 2006, Pages 353–357, https://doi.org/10.1093/beheco/arj037
Close - Share Icon Share
Abstract
Female multiple mating selects for male adaptations that maximize fertilization success in a context of sperm competition. While male mating strategies usually reflect a trade-off between present and future reproduction, this trade-off is largely removed in systems where the maximum number of matings for males is very small. Selection may then favor extreme mechanisms of paternity protection that amount to a maximal investment in a single mating. Males in several arthropod taxa break off parts of their copulatory organs during mating, and it has frequently been suggested that mutilated males can thus secure their paternity. Nevertheless, such a mechanism has rarely been confirmed directly. Here we study the golden orb spider Nephila fenestrata, which has a mating system with potentially cannibalistic, polyandrous females, and males that are often functionally sterile after mating with one female only. We show that males in this species can indeed protect their paternity by obstructing the female's genital openings with fragments of their copulatory organs.
Male animals, especially in species where males provide little or no parental investment, are generally expected to maximize their fitness by mating with several females (Bateman, 1948; Clutton-Brock and Vincent, 1991; Trivers, 1972). There are, however, exceptions to this rule. Monogyny (male monogamy), in the absence of a noticeable parental investment by males, is a rare but taxonomically widespread mating strategy that occurs in mammals (Brotherton and Rhodes, 1996), fish, mollusks, crustaceans (Vollrath, 1998), insects (Moritz and Southwick, 1992) and spiders (Andrade, 1996; Foellmer and Fairbairn, 2003). Theory suggests that monogyny may have evolved in these systems because males attained an advantage through maximally investing in paternity protection (Fromhage et al., 2005; see also Andrade, 1996; Thornhill and Alcock, 1983; Yamamura and Tsuji, 1989). Nevertheless, relatively little is known about the mechanisms by which monogynous males protect their paternity. Notable exceptions include the dwarf antelope Madoqua kirkii, where males engage in mate guarding (Brotherton and Rhodes, 1996), the redback spider Latrodectus hasselti, where males increase their paternity by actively sacrificing themselves to cannibalistic females (Andrade, 1996), and the spider Argiope aurantia, where males die spontaneously during copulation to form whole-body mating plugs (Foellmer and Fairbairn, 2003). In a number of spider and insect species, monogynous males regularly incur damage of their copulatory organs during mating (e.g., Andrade, 1996; Knoflach, 2002; Knoflach and van Harten, 2001; Monnin and Peeters, 1998; Moritz and Southwick, 1992), and it has been suggested that the mutilated males can thus secure their paternity. Nevertheless, the putative effect of copulatory organ damage on paternity has rarely been confirmed directly (but see Snow and Andrade, 2005; Snow et al., 2005).
Here we study the golden orb web spider Nephila fenestrata, where males often damage both of their paired mating organs, the pedipalps, while copulating with a single female (Fromhage and Schneider, 2005a). We hypothesize that pedipalp breakage may prevent female remating by placing a physical barrier inside the female tract (the mating plug hypothesis) or because pedipalp fragments provide a cue by which subsequent suitors are deterred from mating (the nonvirginity cue hypothesis). If an N. fenestrata female mates with two males, the relative number of copulatory insertions achieved per male predicts paternity (Fromhage and Schneider, 2005a). We hence evaluate the significance of male pedipalp damage with respect to the number of insertions achieved by a subsequent suitor.
We also study another aspect of mating behavior that involves physical damage to males. During copulation, N. fenestrata males often cast off (autotomize) some of their anterior legs when attacked by the potentially cannibalistic female. Copulation then continues while the female consumes the legs. We manipulate the opportunity for leg autotomy to assess potential effects on male copulation success and sexual cannibalism.
METHODS
Spiders were F2 offspring derived from 74 individuals collected in 2003 in KwaZulu-Natal, South Africa. Females were housed in separate Perspex frames (60 × 60 × 12 cm), where they built typical orb-webs. They were watered on 6 days per week, fed 5–8 Calliphora flies 2–3 times per week, and weighed on the day after their final molt. Their fixed body size was taken by measuring the tibia-patella length of a foreleg with calipers after the experiment. Males were maintained in individual cups (150 ml) on a diet of Drosophila. On the day after the final molt, each male was weighed and the tibia-patella length of a foreleg was measured while he was anesthetized with CO2.
Sexual cannibalism in N. fenestrata reduces male fitness by precluding the possibility of postcopulatory mate guarding (Fromhage and Schneider, 2005a,b). Autotomy might lower the risk of cannibalism because lost legs are consumed by the female, and mating with feeding females has been shown to be less risky for males (Fromhage and Schneider, 2005a). To manipulate the opportunity for leg autotomy, we experimentally removed males' forelegs (“foreleg autotomy” treatment; n = 18), predicting that males without forelegs (the legs most frequently lost in normal matings) should be more prone to cannibalism than others. To control for potential effects of leg loss per se, we used the following control treatments: in the “autotomy control” treatment (n = 17) we removed one leg on either body side, randomly chosen from all legs excluding the forelegs. In the “intact control” treatment (n = 17) we handled males similarly but removed no legs. Each leg to be removed was grasped with tweezers, which lead to its immediate detachment by the male. Males were left for at least 24 h after this procedure before being used in the experiment.
To initiate a trial, we introduced a (control or experimental) male into an upper corner of the female's web. If mating did not occur within 5 h, the male was removed and introduced again 7 days later. This was repeated again if necessary. If mating did still not occur at this stage (N = 3), both male and female were excluded from the experiment. After mating, males were removed from the web and a second, unmanipulated, male was introduced as described above. At this stage, we gave the female a mealworm (Tenebrio molitor), so that the second male could safely initiate mating while the female was feeding. We thus offered favorable mating conditions to second males, which provides a conservative test of the hypothesis that emasculation can prevent female remating. The second male was given 3 h to initiate mating. After this period, he was removed and reintroduced 7 days later for a period of 5 h. We recorded all mating activities as well as occurrences of leg loss and cannibalism. Females did not significantly differ between treatments in mass, age, or fixed body size; nor did first or second males (Kruskal-Wallis tests, all
Males used one or both of their pedipalps for one copulatory insertion each; hence measurements of copulation duration include 1–2 insertions. Copulation was usually preceded by repeated poking of the female's genital openings with the male's pedipalps, which we refer to as pseudocopulation (PC). PCs are much shorter than true copulations, lack rhythmical movements typical of the latter, and do not result in measurable paternity gains (Fromhage and Schneider, 2005a). Because males perform PCs more or less continually between mounting a female and the beginning of copulation, we quantified pseudocopulatory activity as the time a male spent mounted on a female (hereafter PC-duration) before his first copulatory insertion. If no copulation occurred, PC-duration refers to the total time a male spent mounted on a female.
Genital morphology
To detect pedipalp damage arising from mating, the males' pedipalps were visually inspected before and after the experiment, using a 40× stereomicroscope. Similarly, the epigynum of each female (a sclerotized structure where her paired genital openings are located; Figure 2) was inspected after each episode of cohabitation by a male. To detect pedipalp fragments inside insemination ducts and spermathecae, each female's genital tract was excised after her death and macerated with NaOH. Specimens used for electron microscopy were dehydrated in ethanol, dried in a Balzers CPD 030 critical point dryer, coated with gold in a Balzers MED 010 sputtering device, and examined in a Philips XL20 scanning electron microscope.
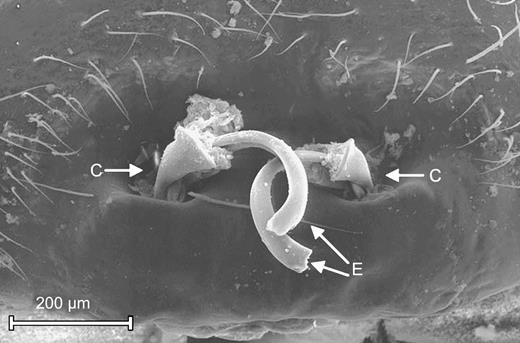
The following details of male morphology are relevant to our study: the conductor is a sclerotized, hook-like process of the pedipalp (Figure 1) that is anchored to the female's genitals during copulation. The embolus (Figure 2) is a flexible tube that is normally hidden within the conductor, but it can be extended during copulation to enter the female's spermatheca.
Mated Nephila fenestrata male, ventral view (legs removed). The conductor (C) is intact on the right pedipalp but missing on the left side (asterisk). Scanning electron microscope image.
Genital region of mated Nephila fenestrata female. Each genital opening is plugged by a conductor (C) and its corresponding embolus (E). Scanning electron microscope image.
Statistical analyses
Statistical analyses were carried out with JMP 5.0. Where possible, data were transformed to fit a normal distribution. We used both parametric and nonparametric tests as appropriate. For details of the tests used, see Sall et al. (2005). Sample sizes can differ between analyses because not all data were available for all trials. Means are given ±SE.
Ethical note
Autotomy is very common in spiders in nature and is considered an adaptive mechanism to prevent predation. Spiders have a special muscular mechanism to detach limbs that have been grasped, and the wound seals naturally with negligible loss of body fluids (Foelix, 1996; Roth VD and Roth BM, 1984). In this study, we invoked the natural autotomy response by grasping a spider's leg. All spiders survived this treatment and resumed locomotory activity immediately afterward.
RESULTS
Pedipalp damage and second males' copulation success
Of 78 pedipalps used by first males in the experiment, 75 (96%) were visibly damaged after use. The most common kind of damage (55 instances, 71%) was a clear-cut loss of the conductor (Figure 1). Conductor loss usually coincided with loss of the corresponding embolus, so that the number of emboli protruding from the epigynum (Figure 2) after mating of the first male was predicted by his number of lost conductors (0–2; Spearman correlation: rs = .86, p < .0001). Alternative kinds of pedipalp damage include blunted conductors, and pedipalps with oddly protruding emboli. Examination of macerated females revealed that broken emboli and conductors within the female tract were not always visible from the outside. The copulatory pattern in N. fenestrata can be either contralateral (i.e., his right pedipalp inserts into her left opening and vice versa) or ipsilateral (i.e., right and left pedipalp insert into the corresponding sides of the female). Therefore it was often impossible to assign the origin of a particular embolus or conductor within a macerated female to a particular male. Hence we decided not to use data obtained through maceration to explain copulatory behavior of second males. Maceration revealed that 15 of 50 females (30%) had more than one embolus inside the same insemination duct. One of these females even had three emboli inside a duct, indicating that one of her sires had used both pedipalps to inseminate the same duct.
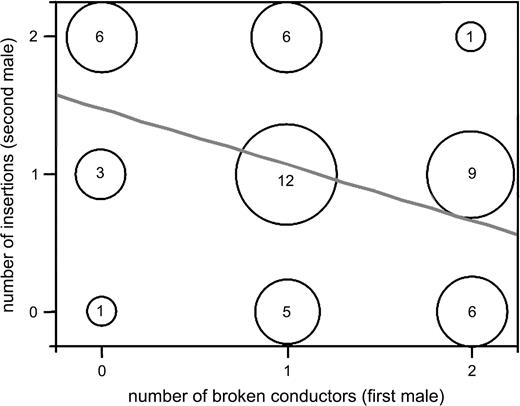
Across treatments, the number of broken conductors of the first male negatively predicted the number of insertions (0–2) achieved by the second male (Figure 3; Spearman correlation: rs = −.4, p = .0042, N = 49). However, a negative correlation between the numbers of insertions achieved by the first and the second male (as reported in Fromhage and Schneider, 2005) was marginally nonsignificant (Spearman correlation rs = −.27, p = .057, N = 52). Second males' body size and mass did not predict their number of insertions (Spearman correlations: rs < .04, p > .7, N = 52).
Number of copulatory insertions achieved by the second male as a function of the number of broken conductors of the first male. Frequency of occurrence is reflected by marker size and given numerically within each circle. Regression line included for display purpose only.
Pseudocopulation
Across treatments, the PC-duration was much shorter for first (5.68 ± 1.33 min) than second males (38.16 ± 7.85 min; Wilcoxon matched-pairs signed-rank test: T = −332.5, p < .0001). The PC-duration of second males was significantly shorter in trials where a copulation eventually ensued (27.05 ± 7.45 min), compared to trials where the second male did not copulate (80.38 ± 20.7 min; Wilcoxon rank sums test: N1 = 10, N2 = 38, z = 3.09, p = .002). Moreover, PC-duration of second males was positively predicted by the number of conductors lost by first males (Spearman correlation: rs = .66, p < .0001).
Depending on whether pedipalp fragments are mating plugs or nonvirginity cues, PC may represent unsuccessful copulation attempts or be a mechanism to assess female status (cf. Suter, 1990). Hence, patterns of PC-duration may allow us to distinguish between the mating plug and the nonvirginity cue hypothesis. Because a female's mating status should be most easily detectable by a male when emboli are protruding from both of her genital openings (as in Figure 2), we compared second males' PC-duration with such females to that observed in the remaining cases. PC-duration with such females (83.21 ± 19.66 min) was significantly longer than with females bearing either one (34.2 ± 10.6 min; Wilcoxon rank sums test: N1 = 12, N2 = 20, z = 2.73, p = .0064) or no protruding embolus (9.31 ± 11.85 min; Wilcoxon rank sums test: N1 = 12, N2 = 16, z = 4.05, p < .0001).
Autotomy treatment and first males' copulation success
Although the treatment affected the opportunity for leg loss during mating (Table 1), it did not significantly affect first males' number of insertions, PC-duration, copulation duration, number of lost conductors, the number of emboli protruding from the epigynum after mating, or sexual cannibalism (Table 1).
Effects of autotomy treatment on mating of first males
| Dependent variable . | Foreleg autotomy . | Autotomy control . | Intact control . | Test . |
|---|---|---|---|---|
| Legs lost per survivor | 0.7 ± 0.26 | 1.89 ± 0.42 | 2.57 ± 0.43 | \(\mathrm{{\chi}}_{2}^{2}{=}8.88,\) p < .012 |
| Mounting latency (min) | 118.71 ± 23.15 | 123.82 ± 23.15 | 124.89 ± 23.15 | F2,48 = 0.019, p = .98 |
| PC-duration | 8.19 ± 2.36 | 5 ± 2.29 | 4 ± 2.29 | F2,47 = 0.88, p = .42 |
| Copulation durationa (min) | 100.61 ± 26.52 | 137.18 ± 24.15 | 123.94 ± 23.29 | F2,47 = 0.19, p = .83 |
| Insertions (0–2) | 1.5 ± 0.17 | 1.7 ± 0.11 | 1.59 ± 0.12 | \(\mathrm{{\chi}}_{2}^{2}{=}0.73,\) p < .70 |
| Broken conductors (0–2) | 1.17 ± 0.19 | 1.07 ± 0.18 | 1.13 ± 0.18 | \(\mathrm{{\chi}}_{2}^{2}{=}0.19,\) p < .91 |
| Emboli protruding from epigynum (0–2) | 0.89 ± 0.18 | 0.94 ± 0.18 | 0.88 ± 0.19 | \(\mathrm{{\chi}}_{2}^{2}{=}0.07,\) p < .97 |
| % males cannibalized | 44% (18) | 47% (17) | 18% (17) | G2 = 4.19, p < .12 |
| Dependent variable . | Foreleg autotomy . | Autotomy control . | Intact control . | Test . |
|---|---|---|---|---|
| Legs lost per survivor | 0.7 ± 0.26 | 1.89 ± 0.42 | 2.57 ± 0.43 | \(\mathrm{{\chi}}_{2}^{2}{=}8.88,\) p < .012 |
| Mounting latency (min) | 118.71 ± 23.15 | 123.82 ± 23.15 | 124.89 ± 23.15 | F2,48 = 0.019, p = .98 |
| PC-duration | 8.19 ± 2.36 | 5 ± 2.29 | 4 ± 2.29 | F2,47 = 0.88, p = .42 |
| Copulation durationa (min) | 100.61 ± 26.52 | 137.18 ± 24.15 | 123.94 ± 23.29 | F2,47 = 0.19, p = .83 |
| Insertions (0–2) | 1.5 ± 0.17 | 1.7 ± 0.11 | 1.59 ± 0.12 | \(\mathrm{{\chi}}_{2}^{2}{=}0.73,\) p < .70 |
| Broken conductors (0–2) | 1.17 ± 0.19 | 1.07 ± 0.18 | 1.13 ± 0.18 | \(\mathrm{{\chi}}_{2}^{2}{=}0.19,\) p < .91 |
| Emboli protruding from epigynum (0–2) | 0.89 ± 0.18 | 0.94 ± 0.18 | 0.88 ± 0.19 | \(\mathrm{{\chi}}_{2}^{2}{=}0.07,\) p < .97 |
| % males cannibalized | 44% (18) | 47% (17) | 18% (17) | G2 = 4.19, p < .12 |
Test statistics indicate use of the following tests: G = G-test, χ2 = Kruskal-Wallis test, F = ANOVA.
Test performed on log-transformed data.
Effects of autotomy treatment on mating of first males
| Dependent variable . | Foreleg autotomy . | Autotomy control . | Intact control . | Test . |
|---|---|---|---|---|
| Legs lost per survivor | 0.7 ± 0.26 | 1.89 ± 0.42 | 2.57 ± 0.43 | \(\mathrm{{\chi}}_{2}^{2}{=}8.88,\) p < .012 |
| Mounting latency (min) | 118.71 ± 23.15 | 123.82 ± 23.15 | 124.89 ± 23.15 | F2,48 = 0.019, p = .98 |
| PC-duration | 8.19 ± 2.36 | 5 ± 2.29 | 4 ± 2.29 | F2,47 = 0.88, p = .42 |
| Copulation durationa (min) | 100.61 ± 26.52 | 137.18 ± 24.15 | 123.94 ± 23.29 | F2,47 = 0.19, p = .83 |
| Insertions (0–2) | 1.5 ± 0.17 | 1.7 ± 0.11 | 1.59 ± 0.12 | \(\mathrm{{\chi}}_{2}^{2}{=}0.73,\) p < .70 |
| Broken conductors (0–2) | 1.17 ± 0.19 | 1.07 ± 0.18 | 1.13 ± 0.18 | \(\mathrm{{\chi}}_{2}^{2}{=}0.19,\) p < .91 |
| Emboli protruding from epigynum (0–2) | 0.89 ± 0.18 | 0.94 ± 0.18 | 0.88 ± 0.19 | \(\mathrm{{\chi}}_{2}^{2}{=}0.07,\) p < .97 |
| % males cannibalized | 44% (18) | 47% (17) | 18% (17) | G2 = 4.19, p < .12 |
| Dependent variable . | Foreleg autotomy . | Autotomy control . | Intact control . | Test . |
|---|---|---|---|---|
| Legs lost per survivor | 0.7 ± 0.26 | 1.89 ± 0.42 | 2.57 ± 0.43 | \(\mathrm{{\chi}}_{2}^{2}{=}8.88,\) p < .012 |
| Mounting latency (min) | 118.71 ± 23.15 | 123.82 ± 23.15 | 124.89 ± 23.15 | F2,48 = 0.019, p = .98 |
| PC-duration | 8.19 ± 2.36 | 5 ± 2.29 | 4 ± 2.29 | F2,47 = 0.88, p = .42 |
| Copulation durationa (min) | 100.61 ± 26.52 | 137.18 ± 24.15 | 123.94 ± 23.29 | F2,47 = 0.19, p = .83 |
| Insertions (0–2) | 1.5 ± 0.17 | 1.7 ± 0.11 | 1.59 ± 0.12 | \(\mathrm{{\chi}}_{2}^{2}{=}0.73,\) p < .70 |
| Broken conductors (0–2) | 1.17 ± 0.19 | 1.07 ± 0.18 | 1.13 ± 0.18 | \(\mathrm{{\chi}}_{2}^{2}{=}0.19,\) p < .91 |
| Emboli protruding from epigynum (0–2) | 0.89 ± 0.18 | 0.94 ± 0.18 | 0.88 ± 0.19 | \(\mathrm{{\chi}}_{2}^{2}{=}0.07,\) p < .97 |
| % males cannibalized | 44% (18) | 47% (17) | 18% (17) | G2 = 4.19, p < .12 |
Test statistics indicate use of the following tests: G = G-test, χ2 = Kruskal-Wallis test, F = ANOVA.
Test performed on log-transformed data.
DISCUSSION
Pedipalp damage in first males affected the behavior of second males such that the latter spent more time performing PCs but achieved fewer copulatory insertions. These findings suggest that pedipalp fragments reduced female remating by presenting a physical barrier to future copulations, rather than by revealing her mating status to subsequent suitors.
In contrast, we found no support for a role of male leg autotomy in mediating mating success or in preventing sexual cannibalism.
Males in many animal species employ mating plugs as a mechanism to prevent female remating. Mating plugs can be formed of secretory substances, as occurs in mammals (Gomendio et al., 1998), spiders (Suhm et al., 1996), and insects (Sauter et al., 2001). Moreover, it has been suggested that detached male mating organs may function as mating plugs in spiders (e.g., Berendonck and Greven, 2002; Knoflach, 2002; Knoflach and van Harten, 2001; Schneider et al., 2005) and insects (Kamimura and Matsuo, 2001; van Veen and Sommeijer, 2000). To validate the latter view, however, it is not sufficient to show that male parts remain attached to the female after mating. For example, Winston (1987) argued that detached male genitalia in the honey bee may reduce sperm flow from the queen's vagina, rather than preventing multiple matings. Similarly, Schneider et al. (2001) found no evidence for broken pedipalp parts preventing female remating in a congener of our study species, Nephila plumipes. Perhaps the clearest evidence reported so far for broken copulatory organs acting as mating plugs exists for the spider L. hasselti, where males break off a small part of the embolus, the apical sclerite, inside the female tract (Snow and Andrade, 2005; Snow et al., 2005). Although the apical sclerite in L. hasselti does not prevent other males from copulating into the same genital opening, it does affect paternity by interfering with sperm transfer from the copulatory duct to the spermatheca (Snow et al., 2005; see also Berendonck and Greven, 2002).
In the present study, the number of conductors lost by the first male negatively predicted the number of pedipalp insertions achieved by the second male. Because paternity in N. fenestrata is determined by the relative number of insertions of each male (Fromhage and Schneider, 2005a), this finding suggests that pedipalp damage protects the paternity of first males. However, female remating might be prevented either because pedipalp fragments physically obstruct her openings (the mating plug hypothesis) or because they reveal her mating status to the successor who then refrains from mating (the nonvirginity cue hypothesis). In the former case, PCs performed by second males could be unsuccessful copulation attempts. This interpretation is supported by a positive relationship between pedipalp damage of first males and PC-duration of second males, as would be expected if more mating plugs make subsequent copulations increasingly difficult.
Alternatively, if pedipalp fragments are nonvirginity cues, PCs may be a mechanism of mate assessment (cf. Suter, 1990). However, this interpretation is inconsistent with the pattern of pseudocopulatory activity observed in our study: although assessment of female mating status should be relatively easy when pedipalp fragments are visibly protruding from both of her genital openings (see Figure 2), second males' PCs were particularly protracted in such cases. We conclude that pedipalp fragments are more likely mating plugs than nonvirginity cues, and PCs are unsuccessful copulation attempts rather than a mechanism of mate assessment.
While pedipalp damage of first males substantially reduced mating success of second males (Figure 3), the presence of more than one embolus inside the same insemination duct in a proportion of females shows that the effectiveness of these mating plugs was nevertheless limited. This may reflect constraints on male morphology or may be the result of opposing selection acting on females: if females can benefit from polyandry (see Jennions and Petrie, 2000, for a review), then their genitalia might evolve to counteract monopolization by their first mate. In addition, if the frequent occurrence of mating plugs in N. fenestrata generates a selective advantage for males able to copulate with a female despite the presence of a rival's plugs, then this might further limit the effectiveness of plugging over evolutionary time.
Males will only benefit from placing mating plugs if the advantage in terms of paternity protection outweighs the associated costs. The extent to which N. fenestrata males incur costs of pedipalp damage, however, is unclear. Intuitively one might expect these costs to be high because the simultaneous loss of the anchoring structure (the conductor) and the sperm-transferring structure (the embolus) almost certainly rules out any future use of the pedipalp. On the other hand, the loss of these structures may not be costly once a pedipalp's sperm content has been transferred. In L. hasselti, for example, sperm transfer with a given pedipalp is ruled out by a prior copulation with that pedipalp (Andrade and Banta, 2002) but not by experimentally induced pedipalp damage (Snow et al., 2005). This suggests that postmating male sterility in L. hasselti is more likely a consequence of sperm depletion than of pedipalp damage and that pedipalp damage has negligible costs (Snow et al., 2005). Similarly, sperm depletion has been reported in a congener of our study species, Nephila clavipes, where the male's pedipalps are rarely damaged, but are used for multiple copulations with the same female (Christenson, 1989; Christenson et al., 1985). Although we do not know if sperm depletion occurs in N. fenestrata, we note that we have never seen N. fenestrata males copulating with a previously used pedipalp—even if no physical damage of the pedipalp was evident (Fromhage L, personal observation; see also Fromhage and Schneider, 2005b).
It is worth noting that, under natural conditions, mating plugs in N. fenestrata back up the effect of postcopulatory mate guarding (Fromhage and Schneider, 2005a,b), which operates before a rival can even reach the female. Mate guarding and mating plugs are hence complementary mechanisms by which N. fenestrata males can maximize their paternity with a single female. Fromhage et al. (2005) have shown theoretically that such a (monogynous) mating strategy can evolve only under certain conditions, one of which is a male-biased sex ratio. The present study highlights N. fenestrata as an ideal model organism to test this prediction and thus sets the stage for further research in this field.
We thank G. Uhl for assistance with electron microscopy. S. Nessler, M. Plath, and G. Uhl provided helpful comments on the manuscript. This research was supported by a DFG grant to J.M.S.
References
Andrade MCB,
Andrade MCB, Banta EM,
Berendonck B, Greven H,
Brotherton PNM, Rhodes A,
Christenson TE,
Christenson TE, Brown SG, Wenzl PA, Hill EM, Goist KC,
Clutton-Brock T, Vincent ACJ,
Foellmer MW, Fairbairn DJ,
Fromhage L, Elgar MA, Schneider JM,
Fromhage L, Schneider JM,
Fromhage L, Schneider JM,
Gomendio M, Harcourt AH, Roldán ERS,
Jennions MD, Petrie M,
Kamimura Y, Matsuo Y,
Knoflach B,
Knoflach B, van Harten A,
Monnin T, Peeters C,
Moritz RFA, Southwick EE,
Roth VD, Roth BM,
Sall J, Creighton L, Lehmann A,
Sauter A, Brown MJF, Baer B, Schmid-Hempel P,
Schneider JM, Fromhage L, Uhl G,
Schneider JM, Thomas ML, Elgar MA,
Snow LSE, Abdel-Mesih A, Andrade MCB,
Snow LSE, Andrade MCB,
Suhm M, Thaler K, Alberti G,
Suter RB,
Thornhill R, Alcock J,
Trivers RL,
van Veen JW, Sommeijer MJ,
Author notes
aBiozentrum Grindel, University of Hamburg, Martin-Luther-King, Platz 3, Hamburg D-20146, Germany
bInstitute of Evolutionary Biology and Ecology, University of Bonn, An der mmenburg 1, D-53121 Bonn, Germany