-
PDF
- Split View
-
Views
-
Cite
Cite
Morten C. Moe, Mercy Varghese, Alexandre I. Danilov, Ulf Westerlund, Jon Ramm-Pettersen, Lou Brundin, Mikael Svensson, Jon Berg-Johnsen, Iver A. Langmoen, Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons, Brain, Volume 128, Issue 9, September 2005, Pages 2189–2199, https://doi.org/10.1093/brain/awh574
Close - Share Icon Share
Abstract
It was long held as an axiom that new neurons are not produced in the adult human brain. More recent studies have identified multipotent cells whose progeny express glial or neuronal markers. This discovery may lead to new therapeutic strategies for CNS disorders, either by stimulating neurogenesis in vivo or by transplanting multipotent progenitor cells (MPCs) that have been propagated and differentiated in vitro. The clinical application of such approaches will be limited by the ability of these cells to develop into functional neurons. To facilitate an understanding of mechanisms regulating neurogenesis in the adult human brain, we characterized the developmental processes MPCs go through when progressing to a neuron. Human tissue was harvested during temporal lobe resections because of epilepsy, and cells were cultured as neurospheres. Our findings demonstrate that at an early stage, these cells often stain with neuronal markers without possessing any functional neuronal properties. Over a period of 4 weeks in culture, cells go through characteristic steps of morphological and electrophysiological development towards functional neurons; they develop a polarized appearance with multiple dendrites, whereas the membrane potential becomes more negative and the input resistance decreases [from −48 ± 10 mV/557 ± 85 MΩ (n = 15) between days 7 and 11 to −59 ± 9 mV/380 ± 79 MΩ (n = 9) between days 25 and 38, respectively]. Active membrane properties were first observed on day 7 and consisted of a voltage-gated K+-current. Later in the second week the cells developed voltage-gated Ca2+-channels and fired small Ca2+-driven action potentials. Immature Na+-driven action potentials developed from the beginning of the third week, and by the end of the fourth week the cells fired repetitive action potentials with a completely mature waveform generated by the combined action of the voltage-gated ionic channels INa, IA and IK. After 4 weeks, the newly formed neurons also communicated by the use of GABAergic and glutamatergic synapses. The adult human brain thus harbours MPCs, which have the ability to develop into neurons and in doing this follow characteristic steps of neurogenesis as seen in the developing brain.
Introduction
A hallmark of neurons is the ability to generate action potentials, i.e. excitability (Reh, 2002). Neuronal excitability depends on four factors (Hodgkin and Huxley, 1952; Spitzer et al., 2002): (i) a regenerative process that rapidly produces a large signal, (ii) a threshold, so that only a stimulus significantly greater than noise can initiate an action potential, (iii) a limitation of signal duration that returns the membrane potential to its resting state and (iv) a recovery process that enables reexcitation. The development of excitability shows some dissimilarities between different species and CNS regions, but generally consists of a coordinated succession of changes in passive and active electrical membrane properties, particularly the development of a set of voltage-gated sodium, potassium and calcium channels (for a review see Spitzer et al., 2002).
Neuronal precursors start terminal differentiation when they exit the cell cycle. Acquisition of voltage-gated ionic channels is a crucial part of terminal differentiation, as such channels (i) are required for excitability and (ii) play a fundamental role in morphological and functional maturation of individual neurons and neural networks (Zhang and Poo, 2001; Spitzer et al., 2002; Ben-Ari et al., 2004). Thus, in the developing brain, voltage-gated ionic channels have important functions with respect to cell migration (Komuro and Rakic, 1998; Marin and Rubenstein, 2003), rate of neurite outgrowth (Chemin et al., 2002), axonal targeting (Catalano and Shatz, 1998; Dantzker and Callaway, 1998), modification of action potential waveform (Spitzer et al., 2002), neurotransmitter specification (Borodinsky et al., 2004; Spitzer et al., 2004) and neuronal survival (Mennerick and Zorumski, 2000; Salthun-Lassalle et al., 2004).
During embryogenesis, neurons populating the mammalian neocortex arise from repeated divisions of stem cells (SCs). Glutamatergic projection neurons arise mainly in the ventricular zone (VZ) of the pallium and GABAergic interneurons in the subcortical telencephalon (Anderson et al., 1999; Xu et al., 2004). The VZ persists as a locus of cell proliferation in adult animals and generates neuroblasts wandering by the rostral migratory stream to the olfactory bulb (Lois and Alvarez-Buylla, 1994).
Over the last decade it has become quite well established that also in humans the adult VZ generates new cells, some of which display neuronal antigens after differentiation (Arsenijevic et al., 2001; Johansson et al., 1999; Kirschenbaum et al., 1994; Kukekov et al., 1999; Roy et al., 2000). If these cells have the ability to become synaptically integrated neurons, one may start to speculate in new therapeutic strategies against degenerative diseases by transplantation of multipotent progenitor cells (MPCs) that have been propagated in vitro or by stimulating neurogenesis in vivo (Bjorklund, 2000; Langmoen et al., 2003).
We have earlier shown that monoclonal MPCs harvested from the adult human brain and maintained in vitro differentiate into two functionally separate cell types, one with excitable and one with in-excitable membrane (Westerlund et al., 2003). Future development of therapies based on the use of such cells from the adult human brain, will in the end depend on our understanding of the mechanisms governing proliferation, differentiation and migration of these cells.
Owing to the profound effects voltage-gated ionic channels have on neuronal differentiation and maturation, it is of importance to characterize the sequential steps MPCs from the adult human brain go through when differentiating into functional neurons. In this study, we show that at an early stage cells often stain with neuronal markers without having any functional neuronal properties. Over a period of 4 weeks in culture, however, the cells undergo stepwise acquisition of voltage-gated K+, Ca2+ and Na+ channels, and ultimately not only exhibit the distinct membrane properties of mature neurons, but also connect in a synaptic network as evidenced by spontaneous post-synaptic currents blocked by antagonists against glutamate and GABA. Our results thus also indicate that MPCs from the adult human brain develop into cells fulfilling the criteria for being neurons (Reh, 2002), i.e. excitable cells communicating via synapses.
Materials and methods
Cell culture
Biopsies from the ventricular wall were harvested from 21 temporal lobe specimens obtained during neurosurgery in the cases of medical intractable epilepsy. Tissue harvesting was approved by the Norwegian National Committee for Medical Research Ethics. MRI excluded the presence of tumour, and the patients were screened for infectious diseases. The patients ranged in age from 20 to 44 years (median 28 years). The samples were transported from the operating theatre to the lab in Leibowitz-15 medium (L15) (Invitrogen Corp., Carlsbad, CA) and stored at 4°C.
The tissue was mechanically separated by a scalpel and placed in a medium containing Papain 13.2 U/ml (Sigma, St Louis, MO) for 5 + 5 min. DNAse 200 U/ml (Sigma) was added after 5 min. The dissociated suspension was passed through a 70 μm strainer (BD Biosciences, San Jose, CA), and resuspended as single cells in neurosphere medium (Westerlund et al., 2003). Cells were cultured in Petri dishes or 96/24-well plates (BD Biosciences) at 37°C in 6% CO2 and 20% O2. The cultures were supplemented with bFGF and EGF twice a week, and additional DMEM/F12 was added once a week (Westerlund et al., 2003). The neurospheres were cultured for 3–6 weeks, passaged with Papain/DNAse before the centre became necrotic, and resuspended in 50 : 50 fresh and conditioned neurosphere medium. To ensure strict clonal conditions, single cells were manually isolated with a micromanipulator (Eppendorf, Westbury, NY) and cultured after passage. Differentiation of single cells from neurospheres was induced by adding 2% fetal calf serum (FCS), removal of mitogens and plating on laminin-coated (20 ng/ml) glass bottom dishes (WillCo Wells BV, Amsterdam, The Netherlands) and 4-well glass slides (Nunc, Roskilde, Denmark).
Immunocytochemistry
Immunostaining of cell cultures was performed as previously described (Johansson et al., 1999; Westerlund et al., 2003), with the following primary antibodies and dilutions (rb: rabbit, ms: mouse, gp: guinea pig, goat: gt): glutamate transporter-1 (VGlut-1) (gp, 1 : 10 000, Chemicon, Temecula, CA), MAP-2 (ms, 1 : 200, gift from Prof. P. Morgan, University of Wales), GFAP (rb, 1 : 1000, Dako, Carpinteria, CA), β-III-tubulin (ms, 1 : 1000, Sigma), GAD-65 (rb, 1 : 1000, Chemicon), RIP (ms, 1 : 1000, Chemicon), O4 (ms, 1 : 100, Chemicon), doublecortin (gt, 1 : 100, Santa Cruz, Santa Cruz, CA). TO-PRO-3 (1 : 10 000, Molecular Probes, Eugene, OR) were used for nuclear staining. As secondary antibodies the fluorescent markers Cy3 (1 : 1000, Jackson, West Grove, PA), FITC (1 : 150, Jackson), Alexa Fluor 488 (1 : 500, Molecular Probes) or Cy5 (1 : 200, Jackson Immuno Research Lab Inc, PA) were used.
Electrophysiology
The whole-cell patch-clamp technique was used to examine the neurophysiological properties of individual cells. Cells grown in culture dishes were placed in a recording chamber on the stage of an inverted microscope (Nikon, Tokyo, Japan). The cultures were perfused with DMEM/F12 (Invitrogen Corp.) between 28 and 32°C and bubbled with 95% air and 5% CO2. A Multiclamp 700A amplifier and pClamp 8 software (Axon Instruments, Union City, CA) was used to control pipette potentials and to inject current during recordings. Patch pipettes were pulled from thick-walled borosilicate glass capillaries to resistances of 4–6 MΩ and were filled with pipette solution containing (in mM) K-gluconate 125, HEPES 10, EGTA 10, KCl 5, Mg–ATP 2 and CaCl2 0.2 (pH = 7.3). Lucifer Yellow 0.1% (Molecular Probes, Eugene, OR) was added to the pipette solution to retrospectively identify immunocytochemical markers of the cells tested with electrophysiology. In addition, all cells tested were photographed, and the exact position in the culture dish was marked with a thin water resistant pen to facilitate cell identification. Cells tested electrophysiologically, that were positive for the neuronal markers MAP-2 or β-III-tubulin, were defined as neuron-like cells and included in analysis.
The membrane time constant (τin) and input resistance (Rin) were estimated in current-clamp by the voltage responses of the cells to small injected rectangular hyperpolarizing current pulses of −10 to −30 pA depending on the Rin of the cell. Rin was derived from the linear portion of the current-voltage plot, and τin was calculated by minimizing the squared deviation between the function and the data between 5 and 25 ms of the pulse. The function used was f(t) = Vss − [Vss·exp(t/τin)], where Vss is the steady-state response (Raastad et al., 1998). The voltage-clamp protocol for testing active membrane properties consisted of a 100 ms hyperpolarizing pulse from a holding potential of −70 to −90 mV that preceded each of the depolarizing steps to remove inactivation, followed by 200 ms depolarizing steps with 10 mV increments at 0.5 Hz, taking the membrane potential from −90 to 60 mV. In current-clamp, current pulses (0–0.1 nA, 0.5 Hz) were injected through the patch pipette to examine whether the cells were capable of producing action potentials (APs). Spontaneous synaptic events were recorded during single whole-cell patch-clamp experiments in voltage-clamp at a holding potential of −30 or −70 mV. Spike threshold was defined as the membrane potential at which the slope of the voltage trace increased abruptly during current injection. Spike width was calculated as spike duration at 50% of maximum spike amplitude.
Confocal microscopy
For confocal imaging an inverted microscope (Nikon) with a confocal imaging system (MRC 600, Bio-Rad, Hertfordshire, UK), equipped with an argon ion laser was used. Intracellular Ca2+ ([Ca2+]i) was measured using the acetoxymethyl ester of the two Ca2+-sensitive fluorochromes fluo-3 (fluo-2 AM) and fura red (fura red AM) (Grondahl and Langmoen, 1998) (Molecular Probes). The cells were incubated in DMEM/F12 containing 0.2 μM fluo-3 and 1.8 μM fura red for 20 min before the superfluous dye was washed out. Emitted fluorescent light was detected using two separate photomultiplier tubes at the wavelengths 525–555 nm (fluo-3) and >600 nm (fura red), respectively. One image was acquired every 2 s, and the fluorescence ratio was calculated using the Time Course/Ratiometric Software Module (TCSM) (Bio-Rad).
External test solutions
External test solutions included 0.5 μM of tetrodotoxin (TTX) to block voltage-dependent sodium channels, 500 μM of nickel chloride (NiCl) to block the voltage-gated Ca2+-channels, 500 μM of 4-aminopyridine (4-AP) and 5 mM of tetraethylammonium (TEA) to block potassium currents, 20 μM of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block AMPA/kainate receptors, 15 μM of D-2-amino-5-phosphonovaleric acid (MK-801) to block NMDA receptors and 10 μM of bicuculline to block GABAA receptors (Sigma). The cells were stimulated by pressure puff application of 60 mM of KCl for 10 s.
Statistics
The results are presented as mean ± SEM. Differences were tested with independent-sample t-tests and considered significant when P < 0.05.
Results
Isolation of MPCs
Repeated cell divisions where a single cell ultimately forms a cluster of cells (neurosphere) is shown in Fig. 1A. Biopsies from 21 patients were dissociated into individual cells and grown under conditions promoting neurosphere formation. Primary neurospheres consisting of ∼300 cells were formed after 2–6 weeks in culture in 18 of the 21 samples. Self-renewal capacity was confirmed up to passage 3 (i.e. tertiary spheres) by dissociating spheres into single cells and transferring them to separate wells of a 24-well plate (n = 10) (Fig. 1A, upper left). Of the 240 cells harvested from 10 patients 19 (8%) formed quaternary neurospheres (Fig. 1A). This is comparable with what others have observed after the second passage earlier (Johansson et al., 1999). Attempts on propagating beyond quartenary spheres were in most cases quite unproductive, as the number of newly formed spheres decreased considerably. All the same, multipotency was conserved throughout passages as immunocytochemical staining of progeny of tertiary neurospheres was positive for immature neuronal (doublecortin), neuronal (MAP-2), astrocytic (GFAP) and oligodendroglial (RIP and O4) markers on days 15 of differentiation in all samples tested (n = 5) (Fig. 1B–D).
(A) The generation of a quaternary neurospheres. (B–D) Upon differentiation of tertiary spheres, cells positive for astrocytic (GFAP, green), neuronal [DCX, (B) red; MAP-2, C red] and oligodendrocytic markers [O4, B yellow; RIP, (D) red] develop. DNA is stained with TO-PRO (blue). Scale bars: 15 μm upper left + right, 25 μm lower left, 40 μm lower right (A); 20 μm (B + C); 40 μm (D).
Development of glial and neuronal morphology
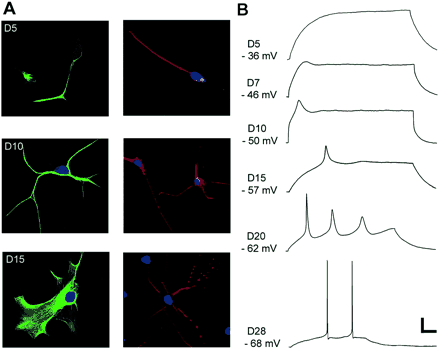
We then followed the development of cells positive for either the glial fibrillary protein GFAP (Fig. 2A, left panel), or the neuronal microtubule-associated protein MAP-2 (Fig. 2A, right panel). This was done by studying cells from secondary and tertiary neurospheres from day 5 (D5) of differentiation to day 28 (D28). During differentiation, GFAP-positive cells were either multipolar (Fig. 2A, D10 left) or more spongiform in appearance resembling protoplasmic astrocytes (Bushong et al., 2004) (Fig. 2, D15 left). MAP-2 positive cells were initially monopolar or bipolar, but later developed multiple extensions, some with local swellings resembling dendritic spines (Yuste and Bonhoeffer, 2004) (Fig. 2, D15 right). On day 15, 68% of the differentiated cells stained for GFAP and 21% stained for MAP-2 (n = 300). None of the cells co-expressed GFAP and MAP.
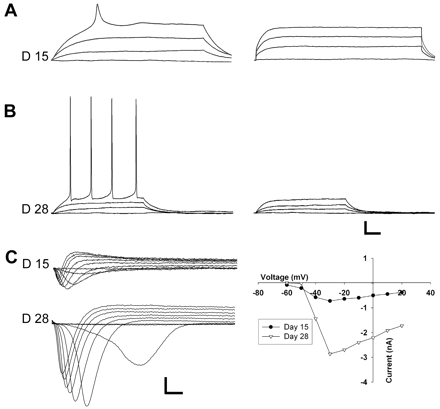
(A) Immunocytochemical staining for glial (GFAP, left panel) and neuronal (MAP-2, right panel) markers at different stages of development. (B) Whole-cell patch-clamp recordings showing responses to a 0.1 nA intracellular current pulse at different stages of differentiation. The pulse duration varied from 300 to 500 ms depending on the maturation of membrane properties. Scale bars: 50 μm (A top panel); 20 μm (A lower panel); 15 mV and 50 ms (B).
Passive membrane properties
Electrophysiological development in neuron-like cells was studied in cells differentiating from secondary and tertiary neurospheres. During the first week of differentiation, all neurone-like cells recorded had a relatively high input resistance, long time constant and low membrane potential (Table 1). Morphological differentiation was accompanied by a gradual decrease in input resistance and increase in membrane potential. Glial-like cells generally had a membrane potential close to –70 mV, an input resistance <100 MΩ and time constant <10 ms (Westerlund et al., 2003).
Changes in membrane properties during neuronal differentiation of MPCs harvested from the adult human brain
. | D7–11 (n = 15) . | D15–18 (n = 10) . | D25–28 (n = 9) . | |||
|---|---|---|---|---|---|---|
| Passive membrane properties | ||||||
| Resting membrane potential (mV) | −48 ± 10 | −59 ± 9* | ||||
| Membrane input resistance (MΩ) | 557 ± 85 | 380 ± 79* | ||||
| Membrane time constant (ms) | 31 ± 11 | 24 ± 7 | ||||
| Active membrane properties | ||||||
| Action potential threshold (mV) | −34 ± 5 | −43 ± 5* | ||||
| Action potential 1/2 width (ms) | 7.3 ± 2.3 | 3.2 ± 0.4* | ||||
| INA (nA) | 1.0 ± 0.2 | 2.6 ± 0.3* | ||||
| IK (nA) | 0.5 ± 0.1 | 1.7 ± 0.4* | ||||
| IA (nA) | 0.3 ± 0.1 | 1.3 ± 0.2* | ||||
. | D7–11 (n = 15) . | D15–18 (n = 10) . | D25–28 (n = 9) . | |||
|---|---|---|---|---|---|---|
| Passive membrane properties | ||||||
| Resting membrane potential (mV) | −48 ± 10 | −59 ± 9* | ||||
| Membrane input resistance (MΩ) | 557 ± 85 | 380 ± 79* | ||||
| Membrane time constant (ms) | 31 ± 11 | 24 ± 7 | ||||
| Active membrane properties | ||||||
| Action potential threshold (mV) | −34 ± 5 | −43 ± 5* | ||||
| Action potential 1/2 width (ms) | 7.3 ± 2.3 | 3.2 ± 0.4* | ||||
| INA (nA) | 1.0 ± 0.2 | 2.6 ± 0.3* | ||||
| IK (nA) | 0.5 ± 0.1 | 1.7 ± 0.4* | ||||
| IA (nA) | 0.3 ± 0.1 | 1.3 ± 0.2* | ||||
The measurements on D25–28 are based only on cells firing repetitive action potentials.
P < 0.05 compared with the earlier stage of differentiation.
Changes in membrane properties during neuronal differentiation of MPCs harvested from the adult human brain
. | D7–11 (n = 15) . | D15–18 (n = 10) . | D25–28 (n = 9) . | |||
|---|---|---|---|---|---|---|
| Passive membrane properties | ||||||
| Resting membrane potential (mV) | −48 ± 10 | −59 ± 9* | ||||
| Membrane input resistance (MΩ) | 557 ± 85 | 380 ± 79* | ||||
| Membrane time constant (ms) | 31 ± 11 | 24 ± 7 | ||||
| Active membrane properties | ||||||
| Action potential threshold (mV) | −34 ± 5 | −43 ± 5* | ||||
| Action potential 1/2 width (ms) | 7.3 ± 2.3 | 3.2 ± 0.4* | ||||
| INA (nA) | 1.0 ± 0.2 | 2.6 ± 0.3* | ||||
| IK (nA) | 0.5 ± 0.1 | 1.7 ± 0.4* | ||||
| IA (nA) | 0.3 ± 0.1 | 1.3 ± 0.2* | ||||
. | D7–11 (n = 15) . | D15–18 (n = 10) . | D25–28 (n = 9) . | |||
|---|---|---|---|---|---|---|
| Passive membrane properties | ||||||
| Resting membrane potential (mV) | −48 ± 10 | −59 ± 9* | ||||
| Membrane input resistance (MΩ) | 557 ± 85 | 380 ± 79* | ||||
| Membrane time constant (ms) | 31 ± 11 | 24 ± 7 | ||||
| Active membrane properties | ||||||
| Action potential threshold (mV) | −34 ± 5 | −43 ± 5* | ||||
| Action potential 1/2 width (ms) | 7.3 ± 2.3 | 3.2 ± 0.4* | ||||
| INA (nA) | 1.0 ± 0.2 | 2.6 ± 0.3* | ||||
| IK (nA) | 0.5 ± 0.1 | 1.7 ± 0.4* | ||||
| IA (nA) | 0.3 ± 0.1 | 1.3 ± 0.2* | ||||
The measurements on D25–28 are based only on cells firing repetitive action potentials.
P < 0.05 compared with the earlier stage of differentiation.
Active membrane properties
The ability to fire APs was tested in current-clamp mode in cells differentiating from secondary and tertiary neurospheres. A total of 14 cells were recorded from during the first week of differentiation. All of these had a completely passive membrane (Fig. 2B, D5). The first active membrane response observed consisted of an apparent small depolarization, ‘depolarizing hump’, evoked by positive current pulses (Fig. 2B, D7). Cells recorded during the second week of differentiation (n = 15) showed either a ‘depolarizing hump’, or a small action potential (Fig. 2B, D10). The former was first observed on day 7, and the latter in the second week.
During the third week, the neuron-like cells developed a more distinct action potential (Fig. 2B, D15) and eventually repetitive firing (Fig. 2B, D20). The action potentials were still broad, and of relatively low amplitude and could not clearly be distinguished from action potentials sometimes seen in developing glial cells (Sontheimer et al., 1992). Between days 25 and 28 most neuron-like cells developed the short-lasting, low-threshold, high-amplitude APs characteristic of mature neurons (Fig. 4B); of 17 tested cells, APs could be elicited in 13, whereas only 3 showed ‘depolarizing humps’ and one failed to show any active membrane properties.
There was a considerable variation in the rate of development. In general, differentiation was faster in more populated areas of the cell cultures, and cells with mature APs were often found in distinct clusters (data not shown). Importantly, differentiated progeny of quaternary spheres were also able to develop into neuron-like cells with mature, repetitive APs (Fig. 2B, D28) (n = 3).
Ionic mechanisms underlying active membrane properties
The ionic nature of the developing APs in neuron-like progeny after differentiation of secondary and tertiary spheres was investigated using specific ionic channel blockers. The first active membrane properties were detected on day 7 (Fig. 3A and B). As described above, this was observed as a small ‘depolarizing hump’ in current-clamp (Fig. 2B, D7 and Fig. 3B), but as an outward current in voltage-clamp (data not shown). It was observed in eight cells recorded during days 7–9 and was in all cases abolished by the K+-channel blockers 4-AP and TEA (Fig. 3B, right), indicating that it is accounted for by outward movement of K+-ions.
(A) Progeny of an adult human multipotent progenitor after 7 days of differentiation (D7), filled with lucifer yellow during patch-clamp recordings (left, yellow), that also stains for the neuronal marker β-III-tubulin (right, red). DNA is stained with TO-PRO-3 (blue). (B) Injection of positive current (0–100 pA, 500 ms) during patch-clamp recordings from the same cell only revealed a depolarizing shift in the membrane potential that sagged to a more hyperpolarized potential during the current pulse. This ‘hump’ was abolished by the K+-channel blockers 0.5 mM 4-AP and 5 mM TEA (right panel). (C) A cell on D10 responded to positive current with a potential that was partly blocked by 500 μM NiCl (middle panel), indicating Ca2+ dependence. The residual ‘hump’ was abolished by 0.5 μM 4-AP and 5 mM TEA. (D) Pressure puff application of KCl 60 mM for 10 s to the peripheral process of a neuron-like cell loaded with fura red and fluo-3 on D10. KCl evoked a rapid elevation in [Ca2+]i that propagated from the application site at the peripheral process (image 2), progressing through the soma and reaching a peak (images 3 and 4) before slowly returning towards baseline (images 5 and 6). A typical response in [Ca2+]i is plotted as the fluorescence intensity ratio of fura red and fluo-3 against time (right). Scale bars: 20 μm (A); 30 mV and 120 ms (B and C).
The cell in Fig. 3 A and B was filled with lucifer yellow (Fig. 3A, left) when patch-clamped. Although the cell only revealed a small depolarizing hump during positive current steps (Fig. 3B, left), it stained with the neuronal marker β-III-tubulin (Fig. 3A, right). This shows that a so-called neuronal marker does not necessarily indicate the presence of functioning neurons.
A small AP-like waveform developed during the second week (Fig. 2B, D10 and Fig. 3C, left). This potential was elicited by depolarizing currents at definite thresholds. It was not affected by the specific Na+-channel blocker TTX, but was sensitive to the Ca2+-channel blocker Ni+ (Fig. 3C, middle), indicating that it is generated by voltage-sensitive Ca2+-channels. A small depolarizing hump that remained after Ni+ administration (Fig. 3C, middle), was abolished by the K+-channel blockers 4-AP and TEA (Fig. 3C, right).
To further investigate the presence of voltage-gated Ca2+-channels at this stage, we examined changes in [Ca2+]i by confocal microscopy (Grondahl and Langmoen, 1998). Depolarizations induced by pressure puff application of KCl (60 mM) directly against individual cells (n = 6) caused a transient increase in [Ca2+]i (Fig. 3D, left panel). Repetitive responses could be evoked (Fig. 3D, right). Taken together, these data suggest that the neuron-like cells at this stage express voltage-gated Ca2+ channels, and that they generate Ca2+-dependent action potentials.
From the beginning of the third week, a sharper waveform was elicited at a more negative membrane potential (Fig. 2B, D15 and Fig. 4A, left). This potential was blocked by TTX (Fig. 4A, right). Voltage-clamp recordings revealed an inward current that was activated between −65 and −50 mV (Fig. 4C upper, n = 10) and blocked by TTX (not shown), i.e. a voltage-gated Na+-current (Henderson et al., 1974). The current-voltage plot yielded a bell-shaped curve. The current increased in amplitude with differentiation until the cells had developed mature firing properties (Fig. 4C) (Table 1), reflecting an increase in sodium channel density with maturation.
(A) On D15, a neuron-like cell showed an overshooting, but immature looking AP (left) that was completely abolished by 0.5 μM TTX (right), indicating Na+-dependence. Current pulse: 0–100 pA, 500 ms. (B) Recordings from neuron-like cells during the fourth week of differentiation (left) revealed mature Na+-dependent, low-threshold, repetitive APs blocked by TTX (right). Current pulse: 0-100 pA, 300 ms. (C) Voltage-clamp recordings show development of Na+-currents during development. The inward currents are plotted as a function of the holding potential (right panel). Scale bars: 5 mV and 25 ms (A and B); 0.5 nA and 5 ms (C).
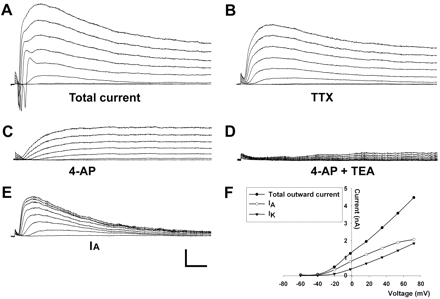
Repetitive, mature APs were seen between days 25 and 28. Utilizing various blockers of voltage-gated ionic channels we were able to isolate the underlying ionic currents (Fig. 5). In voltage-clamp, depolarizing pulses evoked an initial brief inward current (Fig. 5A) that was blocked by TTX (Fig. 5B). Of the 17 neuron-like cells tested during days 25–28, 13 (76%) expressed this Na+-current. This fast inward current was followed by an outward current (Fig. 5A) that persisted after TTX administration (Fig. 5B). It was activated between −50 and −40 mV and increased with depolarization (Fig. 5B). It had two components. First, it had a transient component that peaked within 20 ms and was blocked by bath application of 4-AP (Fig. 5C). This transient current (plotted in Fig. 5E) thus represents an A-type potassium current, IA (Storm, 1990). The amplitude of IA increased significantly with time in culture (Table 1). Secondly, it had a slowly inactivating component that remained after 4-AP (Fig. 5C), but was blocked by TEA (Fig. 5D), thus resembling the delayed rectifier current, IK(DR) (Storm, 1990). The amplitude of IK also increased during development (Table 1). Sixteen of seventeen (94%) D25–28 neuron-like cells expressed both IK(DR) and IA-like currents.
(A) Voltage-clamp recordings in a D20 differentiated cell. The protocol consisted of a prepulse from a holding potential of −70 mV to −90 mV for 100 ms, followed by 200 ms steps of 10 mV to +60 mV. (B) A concentration 0.5 μM of TTX was added to block sodium currents. (C) 4-AP (500 μM) blocked the rapid component of the outward current, resembling the IA current. (D) TEA (5 mM) blocked the remaining IK current. (E) The IA current was calculated by subtracting the 4-AP insensitive from the total K+-currents. (F) The peak outward currents are plotted as the function of the holding potential. Scale bars: 1 nA and 50 ms.
Synaptic transmission
A prerequisite for the communication between neurons is the formation of synaptic contacts. We first tested whether β-III-tubulin positive cells expressed VGlut-1, which is exclusively expressed in glutamatergic neurons (Ni et al., 1995; Takamori et al., 2000), or GAD-65, which is a rate-limiting enzyme in GABA-synthesis and thus is present in GABAergic neurons (Martin and Barke, 1998). Seventeen percent of β-III-tubulin positive cells tested during days 25–28 costained with VGlut-1 (Fig. 6A), whereas GAD-65 (Fig. 6B) was found in 24%.
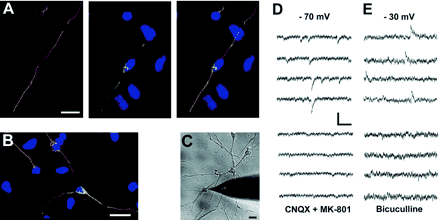
(A) Immunocytochemical detection of VGlut-1 (green) in a cell co-stained with the neuronal marker β-III-tubulin (red), indicating a glutamatergic neuron. DNA is stained with TO-PRO (blue). (B) Co-staining of GAD-65 (green) and β-III-tubulin (red), indicating a GABAergic neuron. DNA is stained with TO-PRO (blue). (D–E) Patch-clamp recordings in D25 neuron-like cells (C) lying in a network revealed spontaneous synaptic currents. At a holding potential of −70 mV, the inward currents were blocked by the glutamate-receptor antagonists CNQX (AMPA/kainate) and MK-801 (NMDA) (D), indicating glutamate-mediated synaptic communication. In another cell at a holding potential of −30 mV, outward currents were blocked by the GABAA-antagonist bicuculline (E), indicating GABAergic synaptic transmission. Scale bars: 20 μm (A–C); 20 pA and 50 ms (D); 15 pA and 200 ms (E).
We then examined whether the expression of these markers reflects the presence of functional glutamatergic and GABAergic synapses. During voltage-clamp of neuron-like cells lying in a network (Fig. 6C, D25) we observed brief spontaneous synaptic currents from a holding potential of −70 mV (Fig. 6D, upper panel). Such spontaneous currents were first detected on day 25, and in 3 of 10 cells tested the currents were blocked by bath application of the glutamate antagonists CNQX and MK-801 (Fig. 6D, lower panel). We then made further recordings from a holding potential of −30 mV, i.e. positive to the reversal potential for the GABA response (Andersen et al., 1980) and observed spontaneous outward currents with a slower time course in 4 of 10 cells tested (Fig. 6E, note the difference in the scale bars). These were blocked by the GABA antagonist bicuculline. These findings indicate that neuron-like cells differentiated from MPCs of adult human brain, establish communication by excitatory and inhibitory synaptic transmission, mediated by presynaptic release of glutamate and GABA. After passage 4 we observed a significantly lower cell-density in the cultures and as a consequence of this a general lack of synaptic events.
Discussion
Using the patch-clamp technique combined with labelling of individual neurones and immunocytochemistry, we show that a self-renewing multipotent cell from the adult human brain proceeds through distinct developmental steps to finally become a mature neuron. Although the pace of maturation differed, our observations may be summarized as follows.
During the first week of differentiation, the cells stained with neuronal markers without exhibiting the functional properties of neurons.
During the second week, the cells first expressed voltage-gated K+-channels that at membrane depolarization resulted in an apparent depolarizing hump, and somewhat later voltage gated Ca2+-channels that produced small action potentials.
Broad, high-threshold, Na+-dependant action potentials appeared at the beginning of the third week and gradually evolved into the short-lasting, low-threshold, repetitive action potentials seen in mature cortical neurones.
By the end of the fourth week, the newly formed neurons exhibited spontaneous GABAergic and glutamatergic post-synaptic currents, indicating that the progeny from a single MPCs had developed into a network of neurons communicating by GABAergic and glutamatergic synapses.
Functional neurogenesis
The ventricular wall tissue samples contained precursors that could be propagated in vitro, and cells were able to differentiate into neurons, astrocytes and oligodendrocytes after both 2 and 3 passages. As each neurosphere developed from a single cell, these observations indicate that the cells fulfil the stem-cell criteria of self-renewal and multipotency (Gage, 2000). The limited replicative ability (4 passages) of the MPCs under our culture conditions is in coherence with other studies of adult human brain progenitors (Johansson et al., 1999; Arsenijevic et al., 2001; Nunes et al., 2003), but contrasts studies on neural progenitors from the foetal brain that have reported extensive self-renewal under similar culture conditions (Piper et al., 2001). As previously suggested (Nunes et al., 2003), the neurosphere-forming cells isolated from the adult human brain thus seem to be a transitional cell type in between SCs and phenotypically commited progenitors.
We next characterized individual cells differentiating after two or more passages. During the first week of development, neuron-like cells had relatively high input resistance and low membrane potential, and showed no signs of active membrane properties. Thereafter, the resting membrane potential gradually increased with development, a phenomenon that has been observed in developing neurons in a variety of experimental preparations (Toda et al., 2000; Wang et al., 2003; Zhou and Hablitz, 1996), although it has been suggested that it may be an artefact owing to the use of patch-clamp electrodes (Zhou and Hablitz, 1996).
The observed decrease in input resistance during development may reflect an increased cell surface area consequent to cell growth. Capacitance also increased, albeit not to a degree that could account for the decrease in input resistance, and as a result the time constant was reduced. The decrease in input resistance is, therefore, most likely a combined effect of growth (increased surface area) and reduced specific resistivity of the cell membrane caused by expression of additional ionic channels (Picken Bahrey and Moody, 2003; Zhou and Hablitz, 1996).
The active current that developed first was not that of a regenerative process characteristic of excitable cells. Although recordings in current-clamp gave the impression of a ‘depolarizing hump’, it was blocked by 4-AP and TEA, and represented an outward K+-current activated by membrane depolarization. It, thus, signifies an early expression of a class of currents that in more mature neurons limit AP duration. It may be important that such currents develop early in order to avoid long-lasting depolarizations and Ca2+-influx when the regenerative currents start to act. It is, however, also of interest to note that the exclusive expression of such a current has been noted in migrating neuronal cells in the chicken hindbrain where it is vital for cell movement, as migration is completely stopped by application of either 4-AP or TEA (Hendriks et al., 1999). It is also expressed in migrating neuroblasts of the rostral migratory stream of adult rats (Belluzzi et al., 2003). This current may thus be expressed in an early stage in order to serve specific developmental purposes before it becomes required for terminating the regenerative potentials in more mature cells.
The next developmental event was typically the expression of a voltage-gated Ca2+-channel as evidenced by an increase in [Ca2+]i on depolarization, broad Ca2+-dependent APs in current clamp and an inward current blocked by Ni2+ in voltage clamp. Calcium channels have numerous functions in the developing CNS (Ben-Ari, 2001; O'Donovan, 1999; Spitzer et al., 2004). Ni2+ typically blocks T-type calcium channels (Perez-Reyes, 2003), which are dominant at the earliest stage (McCobb et al., 1989). This channel is required for neuritogenesis and expression of high-voltage activated calcium channels in neuroblastoma cells (Chemin et al., 2002), and is associated with activity-dependent preservation of neuroprotective intracellular calcium concentration in cultured dopaminergic cells (Salthun-Lassalle et al., 2004).
The regenerative current of the classical AP appeared at the beginning of the third week. The Na+-current was initially weak, and the APs consequently had a broad, low-amplitude waveform. During the third and fourth week the Na+-current increased in parallel with the currents terminating the AP, and the waveform gradually changed into the short-lasting, overshooting action potential seen in mature neurons. In the developing nervous system of lower mammals this process has been related to an increased density of sodium and potassium channels (Gao and Ziskind-Conhaim, 1998; Zhou and Hablitz, 1996). Cells were initially only able to fire single action potentials. This may prevent excessive calcium influx during the prolonged action potential seen in an immature neuron (Zhou and Hablitz, 1996). Immature excitability has previously been observed after 2 weeks differentiation of multipotent neural progenitor cells isolated from the adult human subcortical white matter (Nunes et al., 2003). Voltage-gated Na+-channels and immature APs do, however, also occur in developing glial cells (Sontheimer et al., 1992). In the present study, we identified cells with repetitive, overshooting, low-threshold, short duration APs, signifying that the neuron-like cells had developed all the characteristic of mature neuronal excitability (Spitzer et al., 2002), including a recovery process that enables reexcitation.
Mature neurons typically (i) fire short-lasting, repetitive, low-threshold action potentials and (ii) communicate by synapses. These two properties also define the two most important criteria for determining whether an SC has developed into a functional neurone (Reh, 2002). Following 4 weeks of differentiation, we not only observed mature intrinsic neuronal behaviour, but also spontaneous postsynaptic currents that were blocked by antagonists against GABA and glutamate receptors, respectively. These currents require both spontaneous release of transmitter quanta from presynaptic terminals and postsynaptic receptors. Their presence thus indicates that the cells are integrated in a network of neurons communicating by synapses.
The specificity of differentiated neurons
The cortex has two general groups of neurons, excitatory projection neurons and inhibitory interneurons. The former are glutamatergic and the latter mainly GABAergic. During brain development excitatory projection neurons originate from the VZ (Luskin et al., 1988; Takahashi et al., 1994). Lineage experiments have, however, indicated that interneurons are derived from a separate population of progenitors. Recent studies utilizing topical injections of [3H]thymidine into the embryonic VZ and ganglionic eminence identified the developing striatum as the main source of cortical interneurons (Anderson et al., 2002), and showed that different classes of interneurons originate from distinct regions of the ganglionic eminence (Xu et al., 2004). In the present study we found that the progeny derived from MPCs differentiated into both glutamatergic and GABAergic neurons. This is in keeping with the hypothesis that a part of the GABAergic interneurons of the human cortex originate from the ventricular wall (Letinic et al., 2002), and that new cells in the olfactory bulb express GABA (Winner et al., 2002). Whether cultured MPCs from the human ventricular wall are restricted in differentiation to the cell types found in the cortex is not known. We did, however, observe a number of cells that were both GAD-65 and VGlut1 negative, but still displayed neuronal antigens. These cells may represent neurons with other transmitter specificities, or cells that display neuronal antigens without being true neurons.
Putative clinical implications
The discovery of MPCs in the adult human brain and the emerging technology for expanding and differentiating these cells in vitro may open new possibilities for the treatment of neurodegenerative diseases either by transplanting cells that have been propagated in vitro or by stimulating recruitment of endogenous MPCs. One fundamental mechanism in this scenario is thought to be neuronal replacement, i.e. the ability of the MPCs to replace the neurons lost through the disease process. A more intimate knowledge of how these cells differentiate and behave will improve the prospects for future therapeutic applications. The present paper, where we have characterized the development from progenitor cell to mature neuron, is a modest first approach to such an essential task.
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors
This work was supported by the Swedish Research Council, the Norwegian Foundation for Health and Rehabilitation and The Research Council of Norway.
References
Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Laursen AM. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid.
Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis.
Anderson SA, Kaznowski CE, Horn C, Rubenstein JL, McConnell SK. Distinct origins of neocortical projection neurons and interneurons in vivo.
Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C, et al. Isolation of multipotent neural precursors residing in the cortex of the adult human brain.
Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb.
Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks.
Bjorklund A. Cell replacement strategies for neurodegenerative disorders.
Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons.
Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development.
Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons.
Chemin J, Nargeot J, Lory P. Neuronal T-type alpha 1H calcium channels induce neuritogenesis and expression of high-voltage-activated calcium channels in the NG108-15 cell line.
Dantzker JL, Callaway EM. The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity.
Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons.
Grondahl T, Langmoen IA. Confocal laser scanning microscopy used to monitor intracellular Ca2+ changes in hippocampal CA 1 neurons during energy deprivation.
Henderson R, Ritchie JM, Strichartz GR. Evidence that tetrodotoxin and saxitoxin act at a metal cation binding site in the sodium channels of nerve membrane.
Hendriks R, Morest DK, Kaczmarek LK. Role in neuronal cell migration for high-threshold potassium currents in the chicken hindbrain.
Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve.
Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain.
Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain.
Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations.
Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain.
Langmoen IA, Ohlsson M, Westerlund U, Svensson M. A new tool in restorative neurosurgery: Creating niches for neuronal stem cells.
Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex.
Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain.
Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus.
Martin DL, Barke KE. Are GAD65 and GAD67 associated with specific pools of GABA in brain?
McCobb DP, Best PM, Beam KG. Development alters the expression of calcium currents in chick limb motoneurons.
Mennerick S, Zorumski CF. Neural activity and survival in the developing nervous system.
Ni B, Wu X, Yan GM, Wang J, Paul SM. Regional expression and cellular localization of the Na(+)-dependent inorganic phosphate cotransporter of rat brain.
Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain.
O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system.
Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels.
Picken Bahrey HL, Moody WJ. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex.
Piper DR, Mujtaba T, Keyoung H, Roy NS, Goldman SA, Rao MS, et al. Identification and characterization of neuronal precursors and their progeny from human fetal tissue.
Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus.
Raastad M, Enriquez-Denton M, Kiehn O. Synaptic signaling in an active central network only moderately changes passive membrane properties.
Salthun-Lassalle B, Hirsch EC, Wolfart J, Ruberg M, Michel PP. Rescue of mesencephalic dopaminergic neurons in culture by low-level stimulation of voltage-gated sodium channels.
Sontheimer H, Black JA, Ransom BR, Waxman SG. Ion channels in spinal cord astrocytes in vitro. I. Transient expression of high levels of Na+ and K+ channels.
Spitzer NC, Kingston PA, Manning TJ, Conklin MW. Outside and in: development of neuronal excitability.
Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice.
Takahashi T, Nowakowski RS, Caviness VS, Jr. Mode of cell proliferation in the developing mouse neocortex.
Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons [comment].
Toda H, Takahashi J, Mizoguchi A, Koyano K, Hashimoto N. Neurons generated from adult rat hippocampal stem cells form functional glutamatergic and GABAergic synapses in vitro.
Wang DD, Krueger DD, Bordey A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ.
Westerlund U, Moe MC, Varghese M, Berg-Johnsen J, Ohlsson M, Langmoen IA, et al. Stem cells from the adult human brain develop into functional neurons in culture.
Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb.
Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes.
Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies.
Zhang LI, Poo MM. Electrical activity and development of neural circuits.
Author notes
1Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden, 2Institute for Surgical Research and Department of Neurosurgery, Rikshospitalet, University of Oslo, Norway and 3Neuroimmunology Unit, Centre of Molecular Medicine, Karolinska Institutet, Stockholm, Sweden

![(A) The generation of a quaternary neurospheres. (B–D) Upon differentiation of tertiary spheres, cells positive for astrocytic (GFAP, green), neuronal [DCX, (B) red; MAP-2, C red] and oligodendrocytic markers [O4, B yellow; RIP, (D) red] develop. DNA is stained with TO-PRO (blue). Scale bars: 15 μm upper left + right, 25 μm lower left, 40 μm lower right (A); 20 μm (B + C); 40 μm (D).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/128/9/10.1093/brain/awh574/2/m_awh574f1.gif?Expires=1716320437&Signature=gACdPGnvEfvCQVPcKm211ZZLthY3EBktSf1vDZf3LhWbRP8ckxu55XXNAoBWlcLBtWZIh-v2LI04Dioq9BtH4fQ033BR3hAIa1ft5kfB~VEa1esi7tpi7mluQPCyy8P8B9UPblUg9NUugHEv4tXpyx5xy9tnQQI8hih0m111mVGTXsAiZrcrzx3movHGJn3C0JFCdB3HNxMXAf31SFo5zo0K5vtcN9weHG6F4vMb01QHPT2OFW0UwvYwu9T2dvC-Og8MTA7cmlevZc6YXeaQXZOnB1AlsfhCIg7ZJnwo2LzypHIxvt8oElD0VePnMqPDJQIvdlcodMePR-PgfewOpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![(A) Progeny of an adult human multipotent progenitor after 7 days of differentiation (D7), filled with lucifer yellow during patch-clamp recordings (left, yellow), that also stains for the neuronal marker β-III-tubulin (right, red). DNA is stained with TO-PRO-3 (blue). (B) Injection of positive current (0–100 pA, 500 ms) during patch-clamp recordings from the same cell only revealed a depolarizing shift in the membrane potential that sagged to a more hyperpolarized potential during the current pulse. This ‘hump’ was abolished by the K+-channel blockers 0.5 mM 4-AP and 5 mM TEA (right panel). (C) A cell on D10 responded to positive current with a potential that was partly blocked by 500 μM NiCl (middle panel), indicating Ca2+ dependence. The residual ‘hump’ was abolished by 0.5 μM 4-AP and 5 mM TEA. (D) Pressure puff application of KCl 60 mM for 10 s to the peripheral process of a neuron-like cell loaded with fura red and fluo-3 on D10. KCl evoked a rapid elevation in [Ca2+]i that propagated from the application site at the peripheral process (image 2), progressing through the soma and reaching a peak (images 3 and 4) before slowly returning towards baseline (images 5 and 6). A typical response in [Ca2+]i is plotted as the fluorescence intensity ratio of fura red and fluo-3 against time (right). Scale bars: 20 μm (A); 30 mV and 120 ms (B and C).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/128/9/10.1093/brain/awh574/2/m_awh574f3.gif?Expires=1716320437&Signature=Eh2dfPPMxQrNT4W1C13oWba7SMOfPe1i01QI9XVYL7vVENuZwjUmoCi1S6msUxASfz8j-3nyhrje9OlV3mAp-iBPUnbqOB92I4S6-0mYvw9NYHq2vXQE4tmmBXzMmJS3tDJ6n6K0gVWmnf0LW7tUuo-N2-dsVEkmG3StFwnKtoasEgP8bBkWX0P9cOMBrQIulwEyeL0PkXR-SqNmqSUOKAAdpVgQqmDINDfPH-QxZJGDggOW01WEbXGn18wZfHYRpYwWQHS5BkXaop7JD8HooQMdO6HC2YJBRlVeMKx9dQ1oGsd8khBTprFFOnJtUOvBHKRUOW1IPQOnw~oJwvQm~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)