-
PDF
- Split View
-
Views
-
Cite
Cite
Liat Drucker, Tali Tohami, Shelly Tartakover-Matalon, Victoria Zismanov, Hava Shapiro, Judith Radnay, Michael Lishner, Promoter hypermethylation of tetraspanin members contributes to their silencing in myeloma cell lines, Carcinogenesis, Volume 27, Issue 2, February 2006, Pages 197–204, https://doi.org/10.1093/carcin/bgi209
Close - Share Icon Share
Abstract
Multiple myeloma (MM) cell interactions with their microenvironment modulate acquired drug resistance and disease progression. Indeed, reported aberrant gene methylation underscores the possible role of epigenetic events in MM's molecular profile. Membranal tetraspanins are often inversely correlated with cancer prognosis and metastasis, however mutations were unidentified hitherto. Their promoter characteristics and frequent down-regulation conform to transcriptional silencing by chromatin remodeling. We delineated the baseline expression of select tetraspanins in MM cell lines (RPMI 8226, U266, ARP1, ARK, CAG and EBV transformed ARH77) and fresh bone marrow samples ( n = 9) for the first time and determined reduced expression of CD9, CD81 and absence of CD82. Thus, we aimed to assess their promoter methylation status. Indeed, we established CD9, CD81 and CD82 promoter methylation in MM cell lines employing methyl-specific-PCR of bisulfite modified G-DNA and PCR of G-DNA digested with methylation-sensitive restriction enzyme (Hin6I). Re-transcription of assayed genes in the cell lines following de-methylation [5-aza-2′-deoxycytidine (5-aza-dC)] confirmed the mechanistic significance of methylation to their regulation. Combined de-methylation and de-acetylation [Trichostatin A (TsA)] induced synergistic elevation of CD82 mRNA. We conclude that chromatin remodeling contributes to tetraspanin silencing in MM.
Introduction
The coding potential of the genome lies within the arrangement of its four bases as well as in the distribution of the epigenetically modified 5-methylcytosine ( 1 ). Indeed, DNA methylation patterns evolve in cancer cells and are correlated with the malignant transformation ( 1 ). Aberrant promoter methylation has emerged as a fundamental molecular abnormality leading to transcriptional silencing of select genes.
The simple definition of gene silencing as the conversion of an actively expressed allele to one that is not expressed was initially perceived as an event, which is consistent with numerous studies showing methylated promoters stably inactivated and not expressed ( 2 ). Yet, accumulating data that present variegated methylation patterns, even in clonally derived cell populations, as well as environment and stage-dependent levels of methylation support a more complex evolvement of the gene silencing, such as it being a ‘process’ rather than an ‘event’ ( 2 ).
Several mechanisms of transcriptional repressions by DNA methylation are known and are generally attributed to the assembly of repressive chromatin structures onto DNA by DNA-methylating proteins ( 3 ). Cytosine methylation in CpG dinucleotides is a common hallmark of DNA silencing in many eukaryotes and it is essential for controlling the expression of gene subsets through developmental stages and in all tissues ( 4 ). It is also the most frequently observed hypermethylation mechanism activated in cancer ( 5 ). The consequences of CpG-island methylation during cancer development are increasingly perceived as a series of events beginning with a dramatic drop in transcriptional potential and ending with its complete cessation when the promoter region is inactivated by hypermethylation ( 2 ). Thus, epigenetic silencing of tumor-related genes could be considered one of the two measures necessary for tumor suppressor gene silencing according to Knudson's model.
Chromatin remodeling in multiple myeloma (MM) was reported for genes from different subgroups: cell cycle ( p15 INK4b , p16 INK4a ); apoptosis ( DAP-kinase ; BAD , BAK , BIK and BAX ); cytokine signaling ( SOCS-1 ) and cell-surface receptors ( CD79b , E-cadherin ) ( 6 – 14 ). It was also demonstrated in a number of articles that targeting methylation and acetylation pathways with various agents in vitro and in vivo resulted in an anti-MM activity thus delineating them as potential clinical targets ( 15 – 17 ).
MM cells accumulate in the bone marrow (BM) microenvironment, where they adhere to the extracellular matrix ECM proteins and BM stromal cells (BMSC) ( 18 ). These intensive interactions of the neoplastic cells with their surroundings are modulated by membranal embedded components (i.e. receptors for cytokines and growth factors, adhesion and signaling molecules) ( 19 ). Subsequent changes in the profile of cell adhesion molecules are associated with the migration of tumor cells into the peripheral blood during progressive disease ( 18 ). The functional consequences of their initial adhesion include acquired resistance to apoptosis and augmentation of IL-6 transcription and secretion in BMSC, which mediates both growth and survival for MM cells ( 18 , 19 ). Taken together, the significance of the transmembranal signals to proliferation, survival and drug resistance of the neoplastic cells delineates their circumvention as a primary objective in disease treatment.
The membranal embedded tetraspanin superfamily (transmembrane 4 superfamily) is a group of cell-surface proteins expressed in human as well as primitive organisms and in a wide variety of tissues and cells ( 20 ). Most cells express more than one tetraspanin ( 21 ). By homotypic and heterotypic cellular interactions, they facilitate the spatial organization and localization of multi-protein complexes in distinct membranal microdomains. Particularly, tetraspanins associate with integrins, leukocyte receptors and signaling proteins and modulate fundamental biological functions, such as developmental processes ( 22 , 23 ), signal transduction cascades ( 24 , 25 ), cell migration ( 24 ), cell spreading ( 26 ) and apoptosis ( 27 ). Tetraspanins are often localized at cell–cell junctions thereby influencing the reorganization of actin filaments and they are frequently placed in filopodia and membrane ruffles at the cell-leading edge where their localization and function switch to actin-based motility structures in migrating cells (leukocytes included). They are also involved in the malignant process ( 28 ) (see http://www-ermm.cbcu.cam.ac.uk/01002381h.htm ). CD9 is inversely correlated with the metastatic potential in melanoma; CD63 is expressed in early stages of melanoma; CD82 is a metastasis suppressor in prostate cell carcinoma and the levels of CD9 and CD82 are inversely correlated with metastasis in pancreatic and colorectal cancer. Previous studies addressing the cause of aberrant tetraspanin expression in malignant models found no gene mutations ( 29 ). Transcriptional regulation may be a contributing facet to the modulation of tetraspanin expression levels. In fact, tetraspanin gene promoters are generally GC rich domains deficient of TATA and CAAT boxes ( 21 ) and they comply with the criteria of CpG islands. These promoter characteristics as well as the tetraspanin down regulation in advanced neoplasms comply with the possibility of hypermethylation as an implemented mechanism of transcriptional control. Indeed, the regulatory significance of acetylation to CD9 expression in macrophages was demonstrated ( 30 ).
In our research, we assessed the membranal and cytosolic expression of a select panel of tetraspanins (CD9, CD53, CD63, CD81, CD82, CD151) in MM cell lines. The choice of assayed tetraspanins stemmed from their common expression in leukocytes ( 21 ). Next, we established that the tetraspanins CD9, CD81 and CD82, which are absent in the majority of the MM cell lines, are also characterized with reduced steady-state mRNA levels, implicating that some regulation of gene expression may be attributed to transcription. Our results demonstrate variegated hypermethylation of tetraspanin ( CD9 , CD81 and CD82 ) promoter regions in MM cell lines. We also show that de-methylation can reverse the gene silencing and that a combined application of de-methylating agent and histone deacetylase (HDAC) inhibition has a synergistic effect on CD82 re-expression.
Materials and methods
Cells and cell lines
MM cell lines RPMI 8226 and U266 were purchased from the American Type Culture Collection (Rockville, MD) whereas Prof. Epstein (Little Rock, AR) kindly provided ARP1, ARK and CAG. All were cultured in RPMI 1640 supplemented with 20% heat inactivated fetal calf serum (FCS) and antibiotics (Biological Industries, Beit Haemek, Israel). Epstein Barr virus transformed B-lymphoblastoid cell line ARH 77 was sustained in media containing 20% non-heat inactivated FCS. Twenty-four hours prior to the experiments 3 × 10 6 cells were seeded in 5 ml fresh media. All cell lines will be referred to as MM cell lines henceforth.
Reagents
Antibodies
Phycoerythrin (PE)-coupled monoclonal antibodies such as mouse anti-human CD9, CD53, CD63, CD81, CD82, CD151 and IgG1 Isotype were used. All antibodies were purchased from Becton Dickinson (Ontario, Canada) and administered according to manufacturer's instructions. CD9, CD81 and CD82 will be termed ‘tetraspanins’ from this point forward in generalization. 5-Aza-2′-deoxycytidine (5-aza-dc) and Trichostatin A (TsA) were purchased from Sigma (St Louis, MO) and dissolved in ethanol/acetic acid, respectively.
Methylation-sensitive restriction
Genomic DNA (G-DNA) was extracted from MM cell lines employing the Puregene DNA Isolation kit (Gentra Systems, Minneapolis, MN) according to manufacturer's instructions. G-DNA extracted from normal mononuclear BM cells separated on ficoll gradients served as normal reference. Restriction products of 1 mg G-DNA with methylation-sensitive Hin6I (MBI Fermentas GmbH, St Leon-Rot, Germany) were amplified with primers compatible to unmodified (WT) promoter regions also assayed by methylation-specific PCR (MSP). Primers used are listed in Table I . G-DNA digested with EcoRI (Roche Diagnostics GmbH, Mannheim, Germany) that does not have digestion sites in the promoter regions assessed was used as a positive control for the amplification. Unmethylated G-DNA samples extracted from normal peripheral blood mononuclear cells were used as positive controls for the Hin6I digest and negative control for the PCR. PCR products were visualized on ethidium bromide stained 2% agarose gels with Gel Doc 2000 and the Multi-Analyst software (Bio-Rad laboratories, Hercules, CA).
Primers
| Template . | Forward primer . | Reverse primer . | Temperature (°C) . | Enzyme . |
|---|---|---|---|---|
| CD9WT a | cggcccgggcggggcc | gcgcgcagctgggactggc | 58 | Roche |
| Mm b | tgcggttcgggcggggtc | aacgcgcgcaactaaaactaacg | 58 | Sigma |
| um c | ggtgtggtttgggtggggtt | aaaaacacacacaactaaaactaaca | 55 | Invitrogen |
| RT–PCR | gattgctgtccttgccattgg | ctcatccttgtgggaatatcc | 59 | Sigma |
| CD81WT | cggcggcgaccccaccgc | cctgcggggcgcgggccg | 59 | Sigma |
| Mm | cgacggcggcgattttatcgc | gacctacgaaacgcgaaccg | 55 | Invitrogen |
| Um | gtgatggtggtgattttattgt | acaacctacaaaacacaaaccaa | 54 | Sigma |
| RT–PCR | cccaacaccttctatgtaggc | caccatgctcaggatcatctc | 59 | Sigma |
| CD82WT | gaggagagactccgtagc | ggggctcagtcactcctc | 52 | Invitrogen |
| Mm | atagaggagagatttcgtagc | ccgaaactcaatcactcctc | 52 | Invitrogen |
| Um | atagaggagagattttgtagt | cccaaaactcaatcactccta | 52 | Sigma |
| RT–PCR | ctacaacagcagtcgcgagg | cagctgcctcagtacttggg | 59 | Sigma |
| β actin I | gaccacaccttctacaatgag | gcatacccctcgtagatggg | 59 | Sigma |
| β actin II | gagaccttcaacaccccagc | gctcattgccaatggtgatg | 59 | Sigma |
| Template . | Forward primer . | Reverse primer . | Temperature (°C) . | Enzyme . |
|---|---|---|---|---|
| CD9WT a | cggcccgggcggggcc | gcgcgcagctgggactggc | 58 | Roche |
| Mm b | tgcggttcgggcggggtc | aacgcgcgcaactaaaactaacg | 58 | Sigma |
| um c | ggtgtggtttgggtggggtt | aaaaacacacacaactaaaactaaca | 55 | Invitrogen |
| RT–PCR | gattgctgtccttgccattgg | ctcatccttgtgggaatatcc | 59 | Sigma |
| CD81WT | cggcggcgaccccaccgc | cctgcggggcgcgggccg | 59 | Sigma |
| Mm | cgacggcggcgattttatcgc | gacctacgaaacgcgaaccg | 55 | Invitrogen |
| Um | gtgatggtggtgattttattgt | acaacctacaaaacacaaaccaa | 54 | Sigma |
| RT–PCR | cccaacaccttctatgtaggc | caccatgctcaggatcatctc | 59 | Sigma |
| CD82WT | gaggagagactccgtagc | ggggctcagtcactcctc | 52 | Invitrogen |
| Mm | atagaggagagatttcgtagc | ccgaaactcaatcactcctc | 52 | Invitrogen |
| Um | atagaggagagattttgtagt | cccaaaactcaatcactccta | 52 | Sigma |
| RT–PCR | ctacaacagcagtcgcgagg | cagctgcctcagtacttggg | 59 | Sigma |
| β actin I | gaccacaccttctacaatgag | gcatacccctcgtagatggg | 59 | Sigma |
| β actin II | gagaccttcaacaccccagc | gctcattgccaatggtgatg | 59 | Sigma |
Temp., annealing temperature employed in PCR; enzyme, polymerase used for amplification (Roche—#2140306; Sigma—#D-1086; Invitrogen—#12567-020).
WT, wild-type.
mm, methylated modified.
um, unmethylated modified.
Primers
| Template . | Forward primer . | Reverse primer . | Temperature (°C) . | Enzyme . |
|---|---|---|---|---|
| CD9WT a | cggcccgggcggggcc | gcgcgcagctgggactggc | 58 | Roche |
| Mm b | tgcggttcgggcggggtc | aacgcgcgcaactaaaactaacg | 58 | Sigma |
| um c | ggtgtggtttgggtggggtt | aaaaacacacacaactaaaactaaca | 55 | Invitrogen |
| RT–PCR | gattgctgtccttgccattgg | ctcatccttgtgggaatatcc | 59 | Sigma |
| CD81WT | cggcggcgaccccaccgc | cctgcggggcgcgggccg | 59 | Sigma |
| Mm | cgacggcggcgattttatcgc | gacctacgaaacgcgaaccg | 55 | Invitrogen |
| Um | gtgatggtggtgattttattgt | acaacctacaaaacacaaaccaa | 54 | Sigma |
| RT–PCR | cccaacaccttctatgtaggc | caccatgctcaggatcatctc | 59 | Sigma |
| CD82WT | gaggagagactccgtagc | ggggctcagtcactcctc | 52 | Invitrogen |
| Mm | atagaggagagatttcgtagc | ccgaaactcaatcactcctc | 52 | Invitrogen |
| Um | atagaggagagattttgtagt | cccaaaactcaatcactccta | 52 | Sigma |
| RT–PCR | ctacaacagcagtcgcgagg | cagctgcctcagtacttggg | 59 | Sigma |
| β actin I | gaccacaccttctacaatgag | gcatacccctcgtagatggg | 59 | Sigma |
| β actin II | gagaccttcaacaccccagc | gctcattgccaatggtgatg | 59 | Sigma |
| Template . | Forward primer . | Reverse primer . | Temperature (°C) . | Enzyme . |
|---|---|---|---|---|
| CD9WT a | cggcccgggcggggcc | gcgcgcagctgggactggc | 58 | Roche |
| Mm b | tgcggttcgggcggggtc | aacgcgcgcaactaaaactaacg | 58 | Sigma |
| um c | ggtgtggtttgggtggggtt | aaaaacacacacaactaaaactaaca | 55 | Invitrogen |
| RT–PCR | gattgctgtccttgccattgg | ctcatccttgtgggaatatcc | 59 | Sigma |
| CD81WT | cggcggcgaccccaccgc | cctgcggggcgcgggccg | 59 | Sigma |
| Mm | cgacggcggcgattttatcgc | gacctacgaaacgcgaaccg | 55 | Invitrogen |
| Um | gtgatggtggtgattttattgt | acaacctacaaaacacaaaccaa | 54 | Sigma |
| RT–PCR | cccaacaccttctatgtaggc | caccatgctcaggatcatctc | 59 | Sigma |
| CD82WT | gaggagagactccgtagc | ggggctcagtcactcctc | 52 | Invitrogen |
| Mm | atagaggagagatttcgtagc | ccgaaactcaatcactcctc | 52 | Invitrogen |
| Um | atagaggagagattttgtagt | cccaaaactcaatcactccta | 52 | Sigma |
| RT–PCR | ctacaacagcagtcgcgagg | cagctgcctcagtacttggg | 59 | Sigma |
| β actin I | gaccacaccttctacaatgag | gcatacccctcgtagatggg | 59 | Sigma |
| β actin II | gagaccttcaacaccccagc | gctcattgccaatggtgatg | 59 | Sigma |
Temp., annealing temperature employed in PCR; enzyme, polymerase used for amplification (Roche—#2140306; Sigma—#D-1086; Invitrogen—#12567-020).
WT, wild-type.
mm, methylated modified.
um, unmethylated modified.
Methylation-specific polymerase chain reaction
Bisulfite treatment of 1 µg G-DNA for the conversion of unmethylated cytosine to uracil was performed with a commercially available kit (CpGenome™ Universal DNA Modification Kit, Chemicon International, Temecula, CA). The primers for the methylated modified (mm) and unmethylated modified (um) promoter regions of CD9 , CD81 and CD82 are shown in Table I . Modified DNA (50 ng) was amplified by MSP at least twice with unmodified G-DNA used as a negative control, indicating the specificity of the MSP for modified DNA. In calibration of respective reactions, several polymerases were used and are delineated in Table I along with their respective annealing temperatures. Specificity of MSP products was authenticated by restriction analysis.
De-methylation and HDAC inhibition
Myeloma cells were seeded at a density of 3 × 10 6 /5 ml/25 cm tissue culture flasks and incubated with 10 µM 5-aza-dc or 40 nM TsA at 37°C for 72 or 24 h, respectively. For the combination study, 5-aza-dc was present in the culture for 72 h, and TsA was added for the last 24 h. Cells treated with solvent only (ethanol or acetic acid) were considered a control.
Semi-quantitative reverse transcription PCR
Of the total RNA extracted (Purescript, Gentra Systems) 1 µg was reverse transcribed with Superscript II (Invitrogen, Carlsbad, CA) and Oligo-d(T) 15 primers following standard procedures in a total reaction volume of 20 µl. Multiplex PCR of respective cDNAs (3 µl) and internal control was performed for CD9, CD81 and CD82 with and without 5-aza-dc/TsA treatments. PCR programs were optimized at the logarithmic phase (23 cycles) with simultaneous amplification of the housekeeping β-actin as an internal reference for reaction efficiency. Normalization with β-actin was previously demonstrated in myeloma cell lines as well as in de-methylating treatments repeatedly with reliable reproducible results. The annealing temperature for all reactions was 59°C and the primers are listed in Table I . PCR products were electrophoresed in 2% agarose gels stained with ethidium bromide and visualized with Gel Doc 2000 and Multi-analyst software (Bio-Rad).
Flow cytometry
Cells were harvested, sedimented by centrifugation at 300 g , resuspended in 50 µl PBS and assessed for antigen surface expression by adding the specific antibodies to the sample tube (PE/FITC conjugated) according to manufacturer's instructions. IgG1 matched isotypes were used to exclude nonspecific binding. Fluorescence was analyzed by flow cytometry employing a Coulter Flow Cytometer (FACS) (EPICS-XL, Beckman Coulter, UK). Experiments were repeated 3 times and at least 10 000 events were counted in each FACS analysis. All reaction conditions and flow parameters were standardized. The data are presented as mean ± SE (standard error) calculated for percentages or mean fluorescence intensity (MFI) (arbitrary units) of expressing cells.
Statistical analysis
Student's paired t -tests were employed in the analysis of differences between cohorts. An effect was considered significant when P -value was ≤0.05.
Results
Tetraspanins comply with hypermethylation target criteria
Employing FACS analysis of tetraspanins' (CD9, CD53, CD63, CD81, CD82 and CD151) membranal and cytosolic expression we determined the predominant absence of CD9, CD81 and CD82 in MM cell lines ( Table II ) which is contrary to their widespread distribution in peripheral blood leukocytes (PBLs) ( 30 ). Baseline expression of tetraspanin mRNA in the MM cell lines was determined by semi-quantitative multiplex reverse transcription (RT)–PCR representatively depicted in Figure 1 . The myeloma cell lines exhibited a more restricted expression of CD9, CD81 and CD82 compared with their widespread expression in normal leukocytes and in concordance with their lack of protein expression. All lines lacked mRNA for at least one of the assayed tetraspanins and ARP1 was essentially deficient of all three transcripts. Promoter sequences of CD9 , CD81 and CD82 (gi1832296; gi338677; and gi1832295, respectively) were evaluated and found rich (>50%) in GC content (74, 55, and 66%, respectively). The promoters also feature multiple CpG sites (>7 in a ∼200 bp span) (37, 43 and 12, respectively) and at an elevated frequency compared with the statistically expected rates in a span of >300 bp (1.5, 2.4, 1, respectively), thereby complying with the accepted definition of CpG islands (Obs CpG /Exp CpG > 0.6) ( 31 ). These promoters also lack TATA and CAAT boxes, thus enhancing the probable significance of binding motifs accessibility for transcription factors such as SP1 ( 32 ). Taken together, these tetraspanins fall in with the definition of genomic hot spots for hypermethylation and epigenetic silencing.
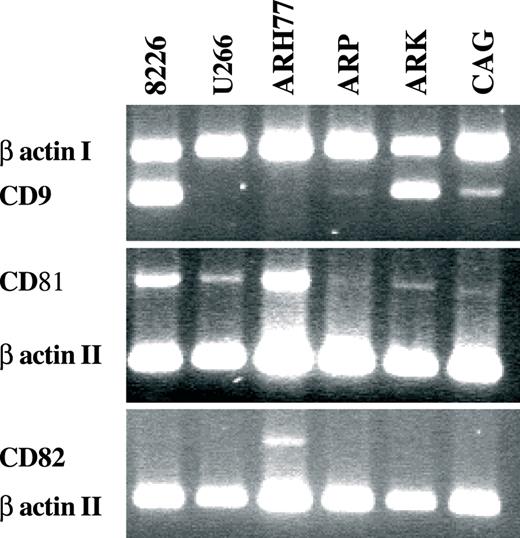
Semi-quantitative RT–PCR of tetraspanins in MM cell lines. Exemplary presentations of multiplex RT reactions of CD9, CD81 and CD82 with β actin were conducted on cell lines (indicated at the top of the figure). CD9 transcript is readily detectable in RPMI 8226 and ARK, and faintly so in CAG. CD81 transcription is suppressed in ARP1 and CAG and weakly detectable in ARK, whereas CD82 mRNA is absent in all lines except ARH77 and somewhat in RPMI 8226. At least duplicate PCR was done to three separate RNA extractions from each cell line with similar results.
| Tetraspanin . | RPMI 8226 . | . | U266 . | . | ARH77 . | . | ARP1 . | . | ARK . | . | CAG . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | ||||||
| CD9 | ++++ | + | — | — | — | — | — | — | + | + | — | — | ||||||
| CD53 | + | — | + | — | + | + | + | — | — | — | + | — | ||||||
| CD63 | + | ++ | ++ | + | — | ++ | + | ++++ | + | +++ | + | ++ | ||||||
| CD81 | ++ | + | — | — | + | + | — | — | — | — | — | — | ||||||
| CD82 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| CD151 | + | — | + | + | + | — | + | + | + | + | + | — | ||||||
| Tetraspanin . | RPMI 8226 . | . | U266 . | . | ARH77 . | . | ARP1 . | . | ARK . | . | CAG . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | ||||||
| CD9 | ++++ | + | — | — | — | — | — | — | + | + | — | — | ||||||
| CD53 | + | — | + | — | + | + | + | — | — | — | + | — | ||||||
| CD63 | + | ++ | ++ | + | — | ++ | + | ++++ | + | +++ | + | ++ | ||||||
| CD81 | ++ | + | — | — | + | + | — | — | — | — | — | — | ||||||
| CD82 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| CD151 | + | — | + | + | + | — | + | + | + | + | + | — | ||||||
The measured parameter was the mean fluorescence intensity (MFI). Expression quantification is presented from the lowest (0 < MFI ≤ 2.5) [+] to the highest (7.5 ≤ MFI) [++++] levels.
These data constitute a section of T.T.'s PhD thesis at Tel-Aviv University, Israel.
| Tetraspanin . | RPMI 8226 . | . | U266 . | . | ARH77 . | . | ARP1 . | . | ARK . | . | CAG . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | ||||||
| CD9 | ++++ | + | — | — | — | — | — | — | + | + | — | — | ||||||
| CD53 | + | — | + | — | + | + | + | — | — | — | + | — | ||||||
| CD63 | + | ++ | ++ | + | — | ++ | + | ++++ | + | +++ | + | ++ | ||||||
| CD81 | ++ | + | — | — | + | + | — | — | — | — | — | — | ||||||
| CD82 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| CD151 | + | — | + | + | + | — | + | + | + | + | + | — | ||||||
| Tetraspanin . | RPMI 8226 . | . | U266 . | . | ARH77 . | . | ARP1 . | . | ARK . | . | CAG . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | Membrane . | Cytoplasm . | ||||||
| CD9 | ++++ | + | — | — | — | — | — | — | + | + | — | — | ||||||
| CD53 | + | — | + | — | + | + | + | — | — | — | + | — | ||||||
| CD63 | + | ++ | ++ | + | — | ++ | + | ++++ | + | +++ | + | ++ | ||||||
| CD81 | ++ | + | — | — | + | + | — | — | — | — | — | — | ||||||
| CD82 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| CD151 | + | — | + | + | + | — | + | + | + | + | + | — | ||||||
The measured parameter was the mean fluorescence intensity (MFI). Expression quantification is presented from the lowest (0 < MFI ≤ 2.5) [+] to the highest (7.5 ≤ MFI) [++++] levels.
These data constitute a section of T.T.'s PhD thesis at Tel-Aviv University, Israel.
Tetraspanins methylation status
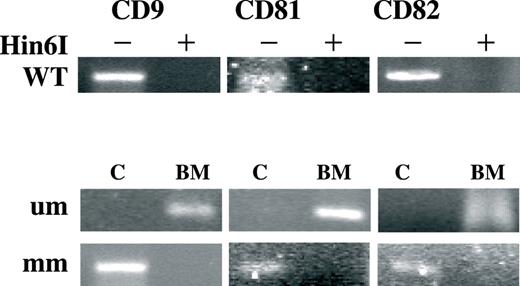
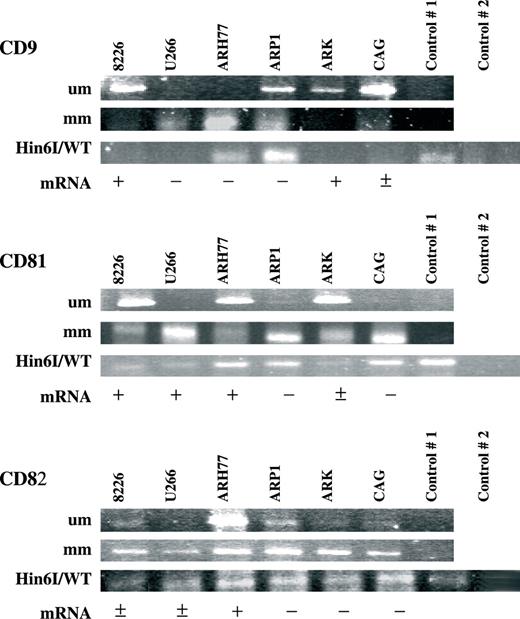
DNA methylation was measured using two different assays. Bisulfite modification followed by MSP allowed a qualitative determination of methylation. MSP was also performed on bisulfite modified G-DNA extracted from normal mononuclear BM cells ( Figure 2 ) and PBLs that express the target proteins and have unmethylated promoter sequences (not shown). In comparison to this normal background, the appearance of a hypermethylated sequence in the promoter regions of the tested MM cell lines constituted a positive signal. Since the bisulfite modification is usually incomplete, we also used unmodified G-DNA from MM cell lines as template for the MSP to rule out any nonspecific amplification. The results of this assay are presented in Figure 3 (top two strips of each figure section). The MSP analysis supported the existence of promoter methylation but its extent varied among the cell lines and genes. It was also evident that there is partial methylation in some of the cases. PCR products with primers matching the methylated and modified sequences (‘mm’ in Figure 3 ) were detected in all assayed MM cell lines for CD81 (6/6) and CD82 (6/6) whereas the examination of CD9 displayed methylation for all lines except ARK (5/6). Assessment of the MSP products with primers matching the unmethylated modified sequences (‘um’ Figure 3 ) underscored the heterogeneous template population in the tetraspanin promoters in the MM cell lines. Confirmation for these findings was achieved by an alternative approach. Hin6I-digested G-DNAs of MM cell lines were used as template for CD9 , CD81 and CD82 promoter sequence amplification. Since Hin6I is a methylation-sensitive restriction enzyme, the PCR template would be available only if all Hin6I restriction sites in between the reaction primers were abolished by CpG methylation and a full-length promoter span was retained. EcoRI-digested G-DNA that lacks restriction sites in the studied promoter regions served as a positive control for PCR and Hin6I-digested normal PBL G-DNA was used both as a positive control for the restriction enzyme and as a negative control for PCR, demonstrating the lack of unspecific amplification. Hin6I digest and amplification of normal BM mononuclear cells' G-DNA demonstrated as did the MSP that normal plasma cells lack methylation of tetraspanins at the sites targeted in this study ( Figure 2 ). Varied quantities of CD81 and CD82 amplification products were detected in all six myeloma cell lines (bottom strip of Figure 3 three sections). PCR products of CD9 were detected in two of the three MM lines that are deficient of CD9 transcript (ARH77, ARP1). Thus, it is evident that tetraspanin promoter methylation is widespread in MM cell lines. RPMI 8226 which had mRNA for all assayed tetraspanins had partial methylation of the three promoters evidenced by the presence of MSP products with mm primers and low levels of PCR products after Hin6I digestion. U266 also displayed methylation of promoter sequences for all three genes evidenced in MSP ( CD9 , CD81 , CD82 ) and PCR of Hin6I-digested template ( CD81 , CD82 ). Again, the extent of methylation was insufficient in blocking transcription of CD81 and CD82 completely. ARH77 is completely deficient of CD9 mRNA expression and indeed our results demonstrate total methylation of the assayed sites. CD81 and CD82 though expressed in ARH77 are also partially methylated.
Methylation status of select tetraspanin promoters in normal BM mononuclear cells. Top strip (marked Hin6I/WT) displays PCR products of wild-type normal BM G-DNA digested (+) or non-digested (−) with methylation-sensitive Hin6I restriction enzyme. Assayed gene promoters are indicated above, respectively. Two bottom strips depict MSP performed on modified samples of normal BM G-DNA or methylated G-DNA from MM cell line as a control (marked ‘C’). Each selected gene promoter (indicated at the top) was assayed with primers matching the bisulfite modified unmethylated DNA sequences (denoted ‘um’) and primers matching the methylated and modified DNA (denoted ‘mm’). The experiments were executed at least three separate times and representative products are presented in this figure.
Methylation status of select tetraspanin promoters in MM cell lines. MSP was performed for each selected gene promoter (marked CD9, CD81 and CD82) with primers matching the bisulfite modified unmethylated DNA sequences (denoted ‘um’ in the top strip) and primers matching the methylated and modified DNA (denoted ‘mm’ in the center strip). MSP of unmodified G-DNA from MM cell lines was included (control #1, respective strips). The bottom strip displays PCR products of wild-type G-DNA digested with methylation-sensitive Hin6I restriction enzyme (marked Hin6I/WT). EcoRI-digested G-DNA that lacks restriction sites in the studied promoter regions served as a positive control for PCR (control #1, respective strip) and Hin6I-digested normal PBL G-DNA was used both as a positive control for the restriction enzyme and as a negative control for PCR, demonstrating the lack of unspecific amplification (control #2). Below the gel-strips of each gene steady-state transcript levels are qualitatively indicated: none (−); present (+); present at very low levels (±). The experiments were executed at least three separate times and representative products are presented in this figure.
In concordance with the lack of CD9, CD81 and CD82 mRNA in ARP1 we demonstrate the presence of methylation of their respective assayed promoter regions. The extent of this methylation is variegated and incomplete except for CD81 . ARK was unmethylated for CD9 and indeed expressed its transcript. CD81 expressed at low levels in ARK was correspondingly semi-methylated and the lack of CD82 in this cell line is supported by its distinctive level of MSP product as well as a PCR product following Hin6I digestion of the reaction template. In CAG, we established the presence of CD81 and CD82 promoter methylation in concordance with their non-detectable steady-state mRNA in this cell line. CD9 was primarily unmethylated in CAG and indeed is characterized with low-level mRNA.
The use of these two strategies enabled the analysis of CpG sites in the primer regions as well as in between them, thereby in compilation screening a broader spectrum of potential target sites ( Table III ). The use of these very different assay methods also substantiates the reliability of our findings by excluding any false results attributed to technique or procedures.
CpG sites assayed
| Gene . | Forward primer . | Reverse primer . | Hin6I . | Overlapping . | Total assayed . | Total present . |
|---|---|---|---|---|---|---|
| CD9 | 4 | 4 | 5 | 2 | 11 | 23 |
| CD81 | 6 | 6 | 8 | 2 | 18 | 37 |
| CD82 | 2 | 2 | 1 | 0 | 5 | 24 |
| Gene . | Forward primer . | Reverse primer . | Hin6I . | Overlapping . | Total assayed . | Total present . |
|---|---|---|---|---|---|---|
| CD9 | 4 | 4 | 5 | 2 | 11 | 23 |
| CD81 | 6 | 6 | 8 | 2 | 18 | 37 |
| CD82 | 2 | 2 | 1 | 0 | 5 | 24 |
CpG sites assayed
| Gene . | Forward primer . | Reverse primer . | Hin6I . | Overlapping . | Total assayed . | Total present . |
|---|---|---|---|---|---|---|
| CD9 | 4 | 4 | 5 | 2 | 11 | 23 |
| CD81 | 6 | 6 | 8 | 2 | 18 | 37 |
| CD82 | 2 | 2 | 1 | 0 | 5 | 24 |
| Gene . | Forward primer . | Reverse primer . | Hin6I . | Overlapping . | Total assayed . | Total present . |
|---|---|---|---|---|---|---|
| CD9 | 4 | 4 | 5 | 2 | 11 | 23 |
| CD81 | 6 | 6 | 8 | 2 | 18 | 37 |
| CD82 | 2 | 2 | 1 | 0 | 5 | 24 |
Reactivating tetraspanin gene expression
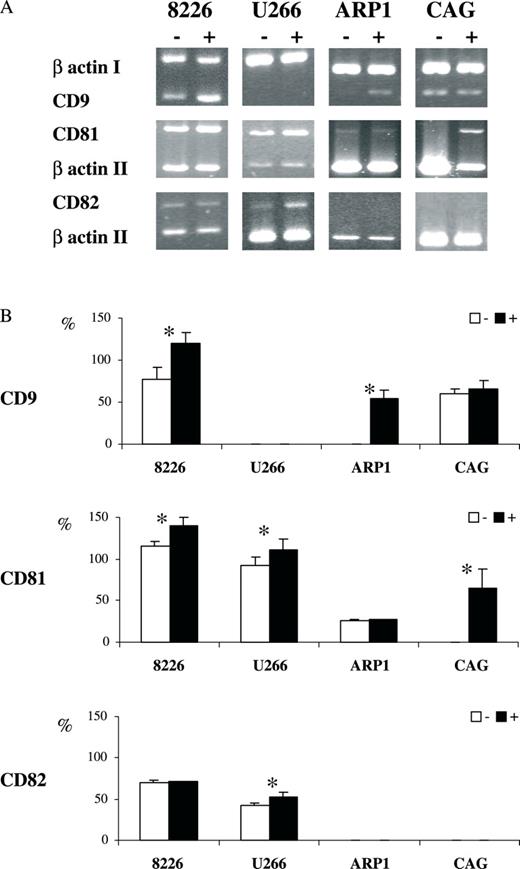
Further evidence for the central role of DNA methylation in the regulation of these tetraspanins came from treatment of cell lines with 5-aza-dc (RPMI 8226, U266, ARP1 and CAG) ( Figure 4A and B ). After a 72-h-treatment with the de-methylating agent, we detected re-expression as well as elevated transcript steady-state level in all three genes. CD9 expression was renewed in ARP1 and enhanced in RPMI 8226 ( P < 0.05); CD81 transcription was renewed in CAG and elevated in RPMI 8226 and U266 ( P < 0.05); CD82 was transcribed at greater levels in U266 ( P < 0.05). Thus, transcription of all three genes was elevated significantly in at least one of the myeloma cell lines (CD9 in RPMI 8226 and CAG; CD81 in RPMI 8226, U266 and CAG; CD82 in U266). It can also be said that all the four MM lines responded to the de-methylating agent with significantly elevated mRNA for at least one tetraspanin (RPMI 8226-CD9, CD81; U266 CD81, CD82; ARP1-CD9; CAG-CD81).
Re-expression of tetraspanins in MM induced by de-methylation. Messenger RNA (mRNA) extracted from MM lines indicated at the top of the figure (8226, U266, ARP1, CAG) without (−)/with (+) 5-aza-dc treatment was assayed for steady-state transcript levels of CD9, CD81 and CD82 by semi-quantitative RT–PCR multiplexed with β-actin used as an internal control (denoted I, II according to primer set used). ( A ) A representative gel electrophoresis presentation of PCR products. ( B ) A quantitative presentation of at least three experiments (mean ± SEM). Statistically significant differences are indicated (asterisk).
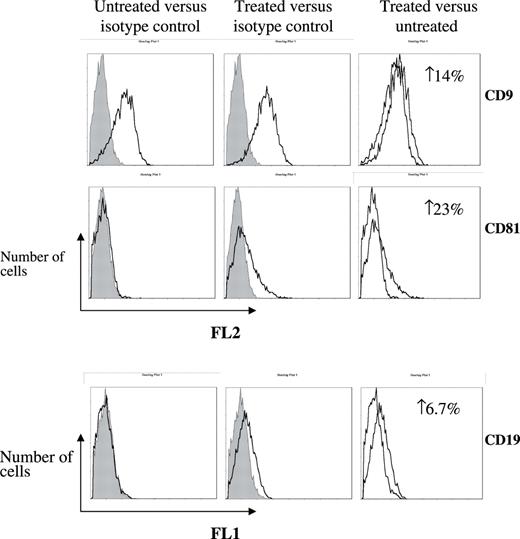
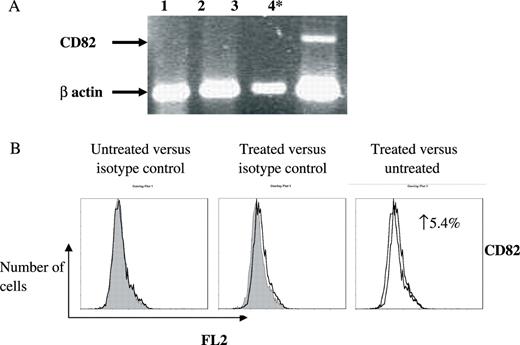
Figure 5 depicts the 5-aza-dc induced increase in membranal expression of CD9 in RPMI 8226 and that of CD81 in U266 treated cells ( P < 0.05). It is established that CD19 is transcriptionally controlled by pax5, which is silenced by methylation in normal plasma cell differentiation ( 33 , 34 ). Thus, CD19 served as a positive control for the de-methylation ( P < 0.05). Taken together, these results indicate that hypermethylation contributes to transcriptional regulation of tetraspanins in MM. The linkage and dominant nature of CpG methylation to histone de-acetylation led us to examine their combined contribution to the gene silencing. Indeed, co-treatment of MM cell lines (ARP1 and CAG) with the de-methylating 5-aza-dc and the HDAC inhibitor TsA resulted in a synergistic re-transcription of CD82 in CAG cells ( P < 0.05) ( Figure 6A and B ).
Membranal re-expression of tetraspanins following de-methylation. RPMI 8226/U266 cells treated with 5-aza-dc or matching solvent (control) were evaluated for membranal expression of tetraspanins by flow cytometry employing monoclonal antibodies for CD9, CD81, CD82, CD19 and matching isotypes. Three separate experiments were conducted and the FACS recorded 10 000 events. These representative histograms depict the number of cells ( y -axis) versus FL1/FL2 fluorescence, respectively ( x -axis). An elevation in membranal expression of CD9 in RPMI 8226 and increased levels of CD19 and CD81 in U266 are indicated as increases of expressing cells (%).
Combined de-methylation and HDAC inhibition induces synergistic re-expression of CD82 transcript in CAG. CAG cells were treated with solvent (lane 1); 5-aza-dc (lane 2); TsA (lane 3); and combined 5-aza-dc TsA (lane 4). The experiment was executed four separate times. ( A ) A representative gel electrophoresis of RT–PCR products. Arrows denote the CD82 transcript and the internal β actin control transcript. Statistically significant differences are indicated (asterisk). ( B ) Membranal expression of CD82 with and without combined 5-aza-dc and TsA treatment was assessed by flow cytometry employing monoclonal antibody and matching isotype. Three separate experiments were conducted and the FACS recorded 10 000 events. These representative histograms depict the number of cells ( y -axis) versus FL2 fluorescence ( x -axis). An elevation of CD82 expression in the treated cells is indicated as increase of expressing cells (%).
Discussion
Previous studies on MM gene expression demonstrated aberrant methylation of several gene promoters often hypermethylated in hematological malignancies ( 35 ). The biological significance of this epigenetic gene regulation is well presented in the prognostic value of p16 promoter methylation to MM progression-free survival ( 8 ). In contrast, normal BM progenitors are generally unmethylated, as are promoters of various genes relevant to hematological neoplasias ( 35 ). It was therefore suggested that detection of gene promoter methylation in neoplasia of hematological origins could be considered an abnormal and cancer-specific phenomenon ( 35 ).
Our research delineates for the first time the baseline expression of select tetraspanins in normal myeloma cell lines. The reduced expression of CD9 and CD81 compared with that of normal B cells and the absence of CD82, which is ubiquitously expressed in human cells, were established. Furthermore, we showed that CD9, CD81 and CD82 are subject to promoter methylation in MM cell lines but not in normal monoclonal BM cells. This was demonstrated by the presence of MSP products with primers matching the methylated promoter sequences and existence of PCR products with Hin6I-digested G-DNAs as templates. The functional contribution of the observed methylation in regulating the transcription of these genes was evidenced by their re-expression following de-methylation. Indeed, we observed enhanced/renewed transcription of each of the three tetraspanins in more than one of the MM lines treated with 5-aza-dc/5-aza-dc and TsA combined. Moreover, at least one of the tetraspanins was re-expressed in each of the 4 lines.
The extent of methylation generally corresponded to the respective genes transcription level. Yet, the MM lines we determined to be methylated in the promoter regions of the assayed tetraspanins repeatedly yielded MSP products with primers matching the unmethylated primer sequences as well. Thus, in the G-DNAs extracted from the various cell lines we recognized a mixed template population indicative of a ‘variegated methylation pattern’ in all three tetraspanins' respective promoters. Previous reports attesting to comparable phenomena in MM have been published ( 7 – 9 ).
Also the heterogeneity in the regulatory impact of the promoter methylation was evident: (i) the partial methylation of a gene promoter silenced its expression in the environment of one cell line yet allowed limited transcription in another. For instance, the CD82 promoter, when partially methylated in both ARH77 and ARP1 cell lines, yielded detectable transcript in the former yet none in the latter. While this could have been attributed to the involvement of additional factors in the regulation of CD82 in ARP1, the gene's re-expression after de-methylating treatment of this cell line proves otherwise. (ii) Different levels of methylation can achieve a comparable regulatory effect on two genes in the same cellular environment. This is exemplified by CD9 and CD81 in ARP1. While no gene transcription was detected for both tetraspanins, the examined CpG sites in CD81 promoter were consistently methylated whereas a mixed outline was determined for CD9 . In a generalization of our results, it could be said that hypermethylation is indeed a contributing regulatory mechanism in tetraspanin transcription. It is most likely a necessary factor in attenuating local chromatin architecture by specifically interacting with methyl CpG binding domain proteins ( 36 ). The complexity of chromatin remodeling with its multiple participants (many as yet unidentified) probably explains why a portion of the transcriptional repression was refractory to de-methylation and HDAC inhibition, as also observed by other groups in various experimental systems ( 36 ).
The findings presented in our study expand the growing list of under-expressed genes in MM in general and especially those regulated, at least to some extent, by epigenetic chromatin remodeling. Despite several publications demonstrating inverse correlation of CD9, CD81 and CD82 mRNA levels and tumor progression and/or metastasis, little work addressed the underlying mechanism of control. Uzawa and colleagues found no CD82 promoter hypermethylation in human oral tumors ( 29 ). Thus, to the best of our knowledge, this is the first comprehensive demonstration of tetraspanin transcriptional regulation and particularly methylation-mediated reduced expression in a malignancy of hematopoietic origin.
Accumulating data obviate the indispensable role of tetraspanins in a variety of biological functions ( 37 – 39 ). In fact, the broad assortments of molecular associations in which they are involved make the dissection of their intertwined functions a challenge. Moreover, it underscores the importance of controlling their expression. The recognition of DNA methylation's involvement in transcriptional regulation of tetraspanins in myeloma marks their re-expression as an achievable therapeutic goal. Yet, it is prudent to keep in mind that expressional regulation of these proteins most probably involves additional mechanisms. The significance of tetraspanin expression or the lack of it in myeloma is yet to be delineated, but it is tempting to speculate that by their modulation of the neoplastic cells' interaction with the microenvironment they may, for instance, affect therapeutic potential via cell adhesion mediated drug resistance ( 40 , 41 ). The proof of such a concept is yet to be obtained but research is underway in our laboratory, which is designed to address this very issue.
We thank Prof. J.Epstein (Multiple Myeloma Research Center, Little Rock, AR) for generously donating ARK, ARP1 and CAG myeloma cell lines to our laboratory. This work was supported by the Tel-Aviv University Research Grant # 06-0138-0221-390005.
Conflict of Interest Statement : None declared.
References
Turker,M. (
Karpf,A.R. and Jones,D.A. (
Ehrlich,M. (
Ng,M.H., Wong,I.H. and Lo,K.W. (
Tasaka,T., Asoc,H., Munker,R., Said,J.W., Berenson,J., Vescio,R.A., Nagai,M., Takahara,J. and Koffler,H.P. (
Mateos,M.V., Gracia-Sanz,R., Lopez-Perez,R. et al. (
Ng,M.H.L., To,K.W., Lo,K.W., Chan,S., Tsang,K.S., Cheng,S.H. and Ng,H.K. (
Ng,M.H.L. (
Pompeia,C., Hodge,D.R., Plass,C., Wu,Y.Z., Marquez,V.E., Kelley,J.A. and Farrar,W.L. (
Galm,O., Yoshikawa,H., Esteller,M., Osieka,R. and Herman,J.G. (
Malone,C.S., Miner,M.D., Doerr,J.R., Jackson,J.P., Jacobson,S.E., Wall,R. and Teitell,M. (
Chim,C.S., Kwong,Y.L., Fung,T.K. and Liang,R. (
Du,H.L., Shi,Y.J., Bu,D.F. and Wu,S.L. (
Mitsiades,N., Mitsiades,C.S., Richardson,P.G. et al . (
Catley,L., Weisberg,E., Tai,Y.T. et al . (
Teoh,G. and Anderson,K.C. (
Epstein,J. and Yaccoby,S. (
Tomlinson,M.G. and Wright,M.D. (
Maecker,H.T., Todd,S.C. and Levy,S. (
Tole,S. and Patterson,P.H. (
Kopczynski,C.C., Davis,G.W. and Goodman,C.S. (
Anton,E.S., Hadjiargyrou,M., Patterson,P.H. and Mathew,W.D. (
Levy,S., Todd,S.C. and Maecker,H.T. (
Koyama,Y., Suzuki,M. and Yoshida,T. (
Beinert,T., Munzing,S., Possinger,K. and Krombach,F. (
Boucheix,C., Duc,G.H.T., Jasmin,C. and Rubinstein,E. (
Uzawa,K., Ono,K., Suzuki,H., Tanak,C., Yakushiji,T., Yamamoto,N., Yokoe,H. and Tanzawa,H. (
Wang,X.Q., Alfaro,M.L., Evans,G.F. and Zuckerman,S.H. (
Tohami,T., Drucker,L., Radnay,J., Shapira,H. and Lishner,M. (
Gardiner-Garden,M. and Frommer,M. (
Borson,D., Lacy,M.Q. and Wettstein,P.J. (
Danbara,M., Kameyama,K., Higashihara,M. and Takagaki,Y. (
Chim,C.S., Liang,R. and Kwong,Y.L. (
Harikrishnan,K.N., Chow,M.Z., Baker,E.K. et al. (
Odintsova,E., Voortman,J., Gilbert,E. and Berditchevski,F. (
Kaji,K. and Kudo,A. (
Tanio,Y., Yamazaki,H., Kunisada,T., Miyake,K. and Hayashi,S.I. (
Damnio,J.S., Cress,A.E., Hazlehurst,L.A., Shtil,A.A. and Dalton,W.S. (
Author notes
1Oncogenetic Laboratory, 2Hematological Laboratory and 3Department of Internal Medicine, Sapir Medical Center, Meir Hospital, Kfar Sava 44281, Israel and 4Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel