-
PDF
- Split View
-
Views
-
Cite
Cite
B. Calvo-Merino, D.E. Glaser, J. Grèzes, R.E. Passingham, P. Haggard, Action Observation and Acquired Motor Skills: An fMRI Study with Expert Dancers, Cerebral Cortex, Volume 15, Issue 8, August 2005, Pages 1243–1249, https://doi.org/10.1093/cercor/bhi007
Close - Share Icon Share
Abstract
When we observe someone performing an action, do our brains simulate making that action? Acquired motor skills offer a unique way to test this question, since people differ widely in the actions they have learned to perform. We used functional magnetic resonance imaging to study differences in brain activity between watching an action that one has learned to do and an action that one has not, in order to assess whether the brain processes of action observation are modulated by the expertise and motor repertoire of the observer. Experts in classical ballet, experts in capoeira and inexpert control subjects viewed videos of ballet or capoeira actions. Comparing the brain activity when dancers watched their own dance style versus the other style therefore reveals the influence of motor expertise on action observation. We found greater bilateral activations in premotor cortex and intraparietal sulcus, right superior parietal lobe and left posterior superior temporal sulcus when expert dancers viewed movements that they had been trained to perform compared to movements they had not. Our results show that this ‘mirror system’ integrates observed actions of others with an individual's personal motor repertoire, and suggest that the human brain understands actions by motor simulation.
Introduction
When we watch someone performing an action, our brains may simulate performance of the action we observe (Jeannerod, 1994). This simulation process could underpin sophisticated mental functions such as communication (Rizzolatti and Arbib, 1998), observational learning (Berger et al., 1979) and socialization (Gallese and Goldman, 1998). Thus it has a major evolutionary benefit.
A specific brain mechanism underlying this process has been suggested. Within the premotor and parietal cortices of the macaque monkey, ‘mirror’ neurons have been recorded which discharge both when the monkey performs an action, and also when observing the experimenter or another monkey performing the same action (di Pellegrino et al., 1992; Gallese et al., 1996; Gallese et al., 2002). A similar mirror system may exist in corresponding areas of the human brain (Decety and Grèzes, 1999; Grèzes and Decety, 2001; Rizzolatti et al., 2001). Buccino et al. (2001) found a somatotopic organization in premotor and parietal cortex when observing movements of different body parts. This somatotopy corresponded to that found when the same body parts are actually moved. The network underlying human action observation seen in functional magnetic resonance imaging (fMRI) includes premotor cortex, parietal areas and the superior temporal sulcus (STS) (Grafton et al., 1996; Rizzolatti et al., 1996; Buccino et al., 2001; Iacoboni et al., 2001), predominantly in the left hemisphere (Decety et al., 1997; Iacoboni et al., 1999; Grèzes et al., 2003). The supplementary motor area and motor cortex are typically not activated, unless an element of movement preparation is also involved, for example in cases of action observation for delayed imitation (Grèzes and Decety, 2001). This might suggest that action observation activates only high-level motor representations, at one remove from actual motor commands. However, transcranial magnetic stimulation (TMS) studies suggest that action observation can directly influence the final cortical stage of action control in the motor cortex. When people observe actions involving a particular group of muscles, responses to transcranial magnetic stimulation (Fadiga et al., 1995; Strafella and Paus, 2000; Baldissera et al., 2001) in those same muscles are specifically facilitated. These results suggest a brain process of motor simulation based on direct correspondence between the neural codes for action observation and for execution.
Some previous studies have suggested that the mirror system activity specifically codes motor actions of a biological agent. First, watching an artificial hand in action evoked much less mirror system activity than watching real hand actions (Perani et al., 2001; Tai et al., 2004). Second, biomechanically impossible actions did not activate the mirror system (Stevens et al., 2000). Finally, Buccino et al. (2004) carried out a study comparing the actions of nonconspecifics, and found that actions belonging to the motor repertoire of the observer were mapped on the observer's motor system. These results suggest that the human mirror system might be sensitive to the degree of correspondence between the observed action and the motor capability of the observer.
However, it remains unclear whether a person's action observation system is precisely tuned to his or her individual motor repertoire. Previous studies of the human mirror system have used a very restricted set of simple actions, based on the primate mirror neurons' responses during grasping (Grafton et al., 1996; Rizzolatti et al., 1996; Grèzes et al., 2003). These studies have reported mirror system activity during observation of grasping, but have not directly tested whether the activity while observing a particular action involves simulating the corresponding motor programme for that action. However, humans have a motor repertoire that far exceeds these simple object-oriented actions, and an apparently unlimited capacity to acquire and perfect new motor skills. As a result, each person's motor repertoire is constrained not only by common musculoskeletal anatomy, but also by the acquired skills that person has learned. A particular action may figure in the motor repertoire of a trained expert but not in the motor repertoire of someone who has not been so trained.
We therefore used acquired motor skills as a powerful way to study the tuning of the brain's mirror mechanisms. We studied groups of people with different acquired motor skills to investigate whether the brain's system for action observation is precisely tuned to the individual's acquired motor repertoire. If this were so, premotor and parietal cortex activity when observing a given action should be stronger in individuals who have learned to perform that action than in those who have not. We tested this hypothesis using a factorial fMRI design in which expert ballet and capoeira dancers watched videos of ballet and capoeira movements. In this way, both groups of expert subjects saw identical action stimuli, but only had motor experience of the actions in their own dance style. We studied these particular expert groups for several reasons. First, both dance styles involve a well-established, distinctive set of movements. Second, many male ballet and capoeira moves are kinematically comparable. Third, dance involves arbitrary, intransitive movements of the whole body. Unlike the grasping tasks previously used to investigate the mirror system in both primate (di Pellegrino et al., 1992; Gallese et al., 1996, 2002) and humans (Grafton et al., 1996; Rizzolatti et al., 1996; Grèzes et al., 2003), dance movements need not involve either external objects or spatial targets locations. Therefore, any differences between groups of dancers must reflect effects of expertise on action observation, and not on the representation of the object or location to which the action is directed. In other acquired motor skills, such as ball games, action observation and object representation might not be easily dissociable. A third group of additional non-expert control subjects was also tested. If differences in action observation activity between the two groups of dancers are truly due to these groups' expertise levels, we predicted that non-expert control subjects should show similar activity when watching either style of dance.
Materials and Methods
Subjects
Ten professional ballet dancers from the Royal Ballet, London, 9 professional capoeira dancers and 10 non-expert control subjects participated. These dance styles were selected because of the kinematic comparability of specific male ballet and capoeira moves. All subjects were right-handed males aged 18–28 with normal vision and no past neurological or psychiatric history. The professional dancers were screened to ensure that they had no training in the other dance style. All gave written informed consent and were paid for their participation. The protocol was approved by the Ethics Committee of the Institute of Neurology, London.
Stimuli and fMRI Task
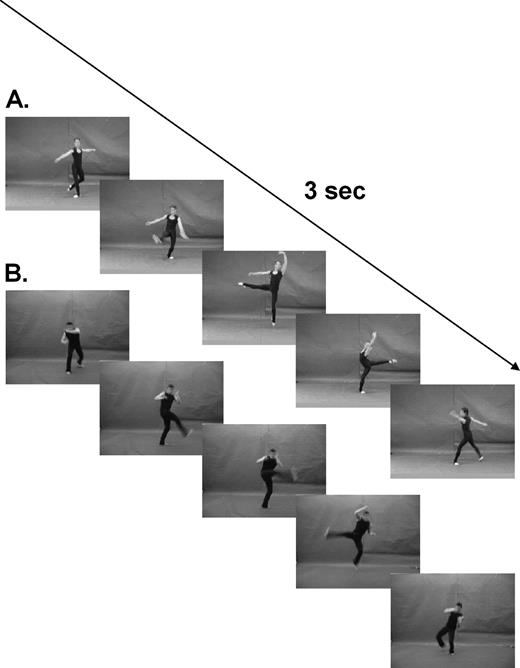
Colour videos of standard classical ballet and capoeira movements were recorded using a digital camera. The movements were performed by ballet and capoeira professionals matched for body shape, appearance and garments, in front of a neutral chromablue background. The performers were naïve regarding the subsequent use of the videos. A professional choreographer approximately matched the individual capoeira moves to classical ballet moves, according to criteria of speed, part of the body employed, body location in space and direction of body movement. This process produced 12 pairs of 3 s video clips. The dancers' faces were blurred to ensure that subjects processed body kinematics, rather than facial or emotional features (see Fig. 1 and online Supplementary material for examples of the videos). The videos were presented on a screen situated outside the scanner which the subject viewed via a mirror (20 × 9 cm) located inside the scanner. During the experiment, each video was repeated four times. Four null events (black screen) were also presented. Stimulus order was randomized. Subjects were instructed to indicate ‘how tiring’ they thought each movement was by pressing one of three keys with three fingers of the right hand. To avoid motor preparation, assignment of buttons to response categories was randomized across trials. Previous training with this response schedule was done outside the scanner with a second set of dance videos.
Stimuli: Colour videos of standard classical ballet and capoeira movements were performed by professional dancers. Twelve different moves of each style (a, ballet; b, capoeira) were matched by a professional choreographer for kinematic features (for examples see videos in the supplementary information online).
Scanning and Data Analysis
The fMRI data were acquired on a 1.5 T Magnetom VISION system (Siemens). Functional images were obtained with a gradient echo-planar sequence using blood oxygenation level-dependent (BOLD) contrast, each comprising 36 contiguous axial slices (2.5 mm thickness). Volumes were acquired continuously with a repetition time (TR) of 3.24 s. A total of 280 scans were acquired for each participant in a single session (15 min), with the first five volumes subsequently discarded to allow for T1 equilibration effects. During fMRI scanning, eye position was monitored on-line by an infrared eye tracker. The data were analysed using a general linear model for event-related design in SPM2 (Wellcome Department of Imaging Neuroscience, London; www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 6.5 release 13. Individual scans were realigned, slice time-corrected, normalized and spatially smoothed by a 6 mm full width at half maximum Gaussian kernel using standard SPM methods. The voxel dimensions of each reconstructed scan were 3 × 3 × 3 mm in the x, y and z dimensions, respectively.
Event-related activity for each voxel, condition and subject was modelled using a canonical haemodynamic response function plus temporal and dispersion derivatives. Statistical parametric maps of the t-statistic (SPM{t}) were generated for each subject and the contrast images were stored.
In a second level random effects analysis, we used a 2 × 3 (stimulus by group) ANOVA model. We constructed an F contrast to test for the group by stimulus interaction, which indicates the extent to which the difference between activity when viewing ballet and when viewing capoeira may vary between groups. Plots of parameter estimates were used to characterize whether the pattern of interaction constitutes an effect of expertise. In order to correct for multiple comparisons in interpreting these results, we used two strategies. First, for areas in the action observation system about which we had a prior anatomical hypothesis, a small volume correction (SVC) with a sphere of 10 mm radius was used according to the coordinates of previous studies. We used Buccino et al. (2001) for premotor and parietal and Grèzes et al. (2004) for posterior STS. Before using SVC, we transformed coordinates given by Buccino et al. (2001) from Talairach space to MNI space (www.mrc-cbu.cam.ac.uk). Second, to reveal unpredicted effects in other areas outside the action observation system, we performed correction for multiple comparisons over the whole brain, with a corrected significance level of P < 0.05. To characterize peak voxels within the activated clusters of interest, we plot contrasts of parameter estimates for each combination of the movement style and subject group. The surviving activated voxels were superimposed on high-resolution magnetic resonance (MR) scans of a standard brain (Montreal Neurological Institute, MNI). In Table 1, we list clusters where SPM{F} for the interaction reached P < 0.001. We have additionally applied an extent threshold of 10 voxels to the data of Table 1, for the sake of brevity. Anatomical identification was performed on cross-sections with reference to the atlas of Duvernoy (1999).
Expertise effects in action observation
| Brain regions . | MNI coordinates . | . | . | Z-score . | ||
|---|---|---|---|---|---|---|
. | x . | y . | z . | . | ||
| Predicted areas(SVC) | ||||||
| L superior precentral gyrus | −27 | −6 | 72 | 3.96 | ||
| R superior precentral gyrus | 30 | −6 | 69 | 3.35 | ||
| R superior parietal lobe | 24 | −69 | 63 | 4.15 | ||
| L posterior intraparietal sulcus/superior parietal lobe | −33 | −45 | 54 | 3.89 | ||
| R intraparietal sulcus/postcentral sulcus | 33 | −42 | 48 | 3.68 | ||
| L precentral gyrus | −54 | 0 | 45 | 3.66 | ||
| L Posterior Superior Temporal Sulcus | −39 | −66 | 36 | 4.04 | ||
| Non-predicted areas (corrected P < 0.05) | ||||||
| Retrosplenial gyrus | 0 | −33 | 36 | 4.98 | ||
| L posterior cingulate gyrus | −3 | −57 | 27 | 5.11 | ||
| R Cingulate gyrus | 3 | 15 | 27 | 5.08 | ||
| Medial frontopolar gyrus | 0 | 39 | −15 | 5.75 | ||
| R parahippocampal gyrus | 33 | −18 | −21 | 4.88 | ||
| Non-predicted areas (uncorrected P < 0.001) | ||||||
| L superior parietal lobe | −21 | −57 | 69 | 3.70 | ||
| L precuneus | −15 | −54 | 51 | 3.88 | ||
| R supramarginal gyrus | 57 | −30 | 48 | 4.00 | ||
| L ventral precentral gyrus | −48 | 3 | 27 | 4.17 | ||
| R parieto-occipital fissure | 21 | −60 | 27 | 3.79 | ||
| R caudate nucleus | 15 | −9 | 18 | 3.92 | ||
| R inferior frontal gyrus | 54 | 36 | 3 | 3.57 | ||
| R head of caudate | 9 | 12 | −6 | 3.45 | ||
| R lateral occipital sulcus | 63 | −48 | −9 | 3.97 | ||
| R lateral occipital sulcus | −51 | −63 | −9 | 3.82 | ||
| L occipital sulcus/middle temporal gyrus | −60 | −39 | −9 | 4.14 | ||
| R lateral orbital frontal gyrus | 33 | 36 | −18 | 3.92 | ||
| L middle temporal gyrus | −60 | −12 | −18 | 3.56 | ||
| L lateral orbital frontal gyrus | −27 | 24 | −27 | 4.61 | ||
| R anterior middle temporal gyrus | 54 | 6 | −30 | 4.46 | ||
| R anterior inferior temporal gyrus | 54 | −12 | −36 | 3.68 | ||
| Brain regions . | MNI coordinates . | . | . | Z-score . | ||
|---|---|---|---|---|---|---|
. | x . | y . | z . | . | ||
| Predicted areas(SVC) | ||||||
| L superior precentral gyrus | −27 | −6 | 72 | 3.96 | ||
| R superior precentral gyrus | 30 | −6 | 69 | 3.35 | ||
| R superior parietal lobe | 24 | −69 | 63 | 4.15 | ||
| L posterior intraparietal sulcus/superior parietal lobe | −33 | −45 | 54 | 3.89 | ||
| R intraparietal sulcus/postcentral sulcus | 33 | −42 | 48 | 3.68 | ||
| L precentral gyrus | −54 | 0 | 45 | 3.66 | ||
| L Posterior Superior Temporal Sulcus | −39 | −66 | 36 | 4.04 | ||
| Non-predicted areas (corrected P < 0.05) | ||||||
| Retrosplenial gyrus | 0 | −33 | 36 | 4.98 | ||
| L posterior cingulate gyrus | −3 | −57 | 27 | 5.11 | ||
| R Cingulate gyrus | 3 | 15 | 27 | 5.08 | ||
| Medial frontopolar gyrus | 0 | 39 | −15 | 5.75 | ||
| R parahippocampal gyrus | 33 | −18 | −21 | 4.88 | ||
| Non-predicted areas (uncorrected P < 0.001) | ||||||
| L superior parietal lobe | −21 | −57 | 69 | 3.70 | ||
| L precuneus | −15 | −54 | 51 | 3.88 | ||
| R supramarginal gyrus | 57 | −30 | 48 | 4.00 | ||
| L ventral precentral gyrus | −48 | 3 | 27 | 4.17 | ||
| R parieto-occipital fissure | 21 | −60 | 27 | 3.79 | ||
| R caudate nucleus | 15 | −9 | 18 | 3.92 | ||
| R inferior frontal gyrus | 54 | 36 | 3 | 3.57 | ||
| R head of caudate | 9 | 12 | −6 | 3.45 | ||
| R lateral occipital sulcus | 63 | −48 | −9 | 3.97 | ||
| R lateral occipital sulcus | −51 | −63 | −9 | 3.82 | ||
| L occipital sulcus/middle temporal gyrus | −60 | −39 | −9 | 4.14 | ||
| R lateral orbital frontal gyrus | 33 | 36 | −18 | 3.92 | ||
| L middle temporal gyrus | −60 | −12 | −18 | 3.56 | ||
| L lateral orbital frontal gyrus | −27 | 24 | −27 | 4.61 | ||
| R anterior middle temporal gyrus | 54 | 6 | −30 | 4.46 | ||
| R anterior inferior temporal gyrus | 54 | −12 | −36 | 3.68 | ||
The table shows transformed Z scores from an SPM{F} for the group by stimulus interaction. The table is divided into three sections. In the first section, we show areas predicted that belong to the action observation system and survive P < 0.05 small volume correction using a 10 mm sphere over coordinates from previous studies [Buccino et al. (2001) for premotor and parietal cortex and Grèzes et al. (2004) for pSTS]. In the second section, we present activations in areas that were not predicted, but that survive correction for multiple comparisons across whole brain at P < 0.05. In the third section, we show areas for which no prediction was made, which are significant at P < 0.001 uncorrected. For the sake of brevity, only activations in excess of 10 voxels are listed in this section of the table. L/R: left and right hemispheres.
Expertise effects in action observation
| Brain regions . | MNI coordinates . | . | . | Z-score . | ||
|---|---|---|---|---|---|---|
. | x . | y . | z . | . | ||
| Predicted areas(SVC) | ||||||
| L superior precentral gyrus | −27 | −6 | 72 | 3.96 | ||
| R superior precentral gyrus | 30 | −6 | 69 | 3.35 | ||
| R superior parietal lobe | 24 | −69 | 63 | 4.15 | ||
| L posterior intraparietal sulcus/superior parietal lobe | −33 | −45 | 54 | 3.89 | ||
| R intraparietal sulcus/postcentral sulcus | 33 | −42 | 48 | 3.68 | ||
| L precentral gyrus | −54 | 0 | 45 | 3.66 | ||
| L Posterior Superior Temporal Sulcus | −39 | −66 | 36 | 4.04 | ||
| Non-predicted areas (corrected P < 0.05) | ||||||
| Retrosplenial gyrus | 0 | −33 | 36 | 4.98 | ||
| L posterior cingulate gyrus | −3 | −57 | 27 | 5.11 | ||
| R Cingulate gyrus | 3 | 15 | 27 | 5.08 | ||
| Medial frontopolar gyrus | 0 | 39 | −15 | 5.75 | ||
| R parahippocampal gyrus | 33 | −18 | −21 | 4.88 | ||
| Non-predicted areas (uncorrected P < 0.001) | ||||||
| L superior parietal lobe | −21 | −57 | 69 | 3.70 | ||
| L precuneus | −15 | −54 | 51 | 3.88 | ||
| R supramarginal gyrus | 57 | −30 | 48 | 4.00 | ||
| L ventral precentral gyrus | −48 | 3 | 27 | 4.17 | ||
| R parieto-occipital fissure | 21 | −60 | 27 | 3.79 | ||
| R caudate nucleus | 15 | −9 | 18 | 3.92 | ||
| R inferior frontal gyrus | 54 | 36 | 3 | 3.57 | ||
| R head of caudate | 9 | 12 | −6 | 3.45 | ||
| R lateral occipital sulcus | 63 | −48 | −9 | 3.97 | ||
| R lateral occipital sulcus | −51 | −63 | −9 | 3.82 | ||
| L occipital sulcus/middle temporal gyrus | −60 | −39 | −9 | 4.14 | ||
| R lateral orbital frontal gyrus | 33 | 36 | −18 | 3.92 | ||
| L middle temporal gyrus | −60 | −12 | −18 | 3.56 | ||
| L lateral orbital frontal gyrus | −27 | 24 | −27 | 4.61 | ||
| R anterior middle temporal gyrus | 54 | 6 | −30 | 4.46 | ||
| R anterior inferior temporal gyrus | 54 | −12 | −36 | 3.68 | ||
| Brain regions . | MNI coordinates . | . | . | Z-score . | ||
|---|---|---|---|---|---|---|
. | x . | y . | z . | . | ||
| Predicted areas(SVC) | ||||||
| L superior precentral gyrus | −27 | −6 | 72 | 3.96 | ||
| R superior precentral gyrus | 30 | −6 | 69 | 3.35 | ||
| R superior parietal lobe | 24 | −69 | 63 | 4.15 | ||
| L posterior intraparietal sulcus/superior parietal lobe | −33 | −45 | 54 | 3.89 | ||
| R intraparietal sulcus/postcentral sulcus | 33 | −42 | 48 | 3.68 | ||
| L precentral gyrus | −54 | 0 | 45 | 3.66 | ||
| L Posterior Superior Temporal Sulcus | −39 | −66 | 36 | 4.04 | ||
| Non-predicted areas (corrected P < 0.05) | ||||||
| Retrosplenial gyrus | 0 | −33 | 36 | 4.98 | ||
| L posterior cingulate gyrus | −3 | −57 | 27 | 5.11 | ||
| R Cingulate gyrus | 3 | 15 | 27 | 5.08 | ||
| Medial frontopolar gyrus | 0 | 39 | −15 | 5.75 | ||
| R parahippocampal gyrus | 33 | −18 | −21 | 4.88 | ||
| Non-predicted areas (uncorrected P < 0.001) | ||||||
| L superior parietal lobe | −21 | −57 | 69 | 3.70 | ||
| L precuneus | −15 | −54 | 51 | 3.88 | ||
| R supramarginal gyrus | 57 | −30 | 48 | 4.00 | ||
| L ventral precentral gyrus | −48 | 3 | 27 | 4.17 | ||
| R parieto-occipital fissure | 21 | −60 | 27 | 3.79 | ||
| R caudate nucleus | 15 | −9 | 18 | 3.92 | ||
| R inferior frontal gyrus | 54 | 36 | 3 | 3.57 | ||
| R head of caudate | 9 | 12 | −6 | 3.45 | ||
| R lateral occipital sulcus | 63 | −48 | −9 | 3.97 | ||
| R lateral occipital sulcus | −51 | −63 | −9 | 3.82 | ||
| L occipital sulcus/middle temporal gyrus | −60 | −39 | −9 | 4.14 | ||
| R lateral orbital frontal gyrus | 33 | 36 | −18 | 3.92 | ||
| L middle temporal gyrus | −60 | −12 | −18 | 3.56 | ||
| L lateral orbital frontal gyrus | −27 | 24 | −27 | 4.61 | ||
| R anterior middle temporal gyrus | 54 | 6 | −30 | 4.46 | ||
| R anterior inferior temporal gyrus | 54 | −12 | −36 | 3.68 | ||
The table shows transformed Z scores from an SPM{F} for the group by stimulus interaction. The table is divided into three sections. In the first section, we show areas predicted that belong to the action observation system and survive P < 0.05 small volume correction using a 10 mm sphere over coordinates from previous studies [Buccino et al. (2001) for premotor and parietal cortex and Grèzes et al. (2004) for pSTS]. In the second section, we present activations in areas that were not predicted, but that survive correction for multiple comparisons across whole brain at P < 0.05. In the third section, we show areas for which no prediction was made, which are significant at P < 0.001 uncorrected. For the sake of brevity, only activations in excess of 10 voxels are listed in this section of the table. L/R: left and right hemispheres.
Results
Functional images were analysed by statistical parametric mapping (SPM2) using a general linear model applied at each voxel across the whole brain. We localized those brain areas that modulated their activity with expertise using an F-test. We defined the expertise effect as the interaction between the three subject groups and the two kinds of dance stimuli. That is, we focused on voxels for which the difference between the responses to the two types of stimuli varied across the three groups of subjects.
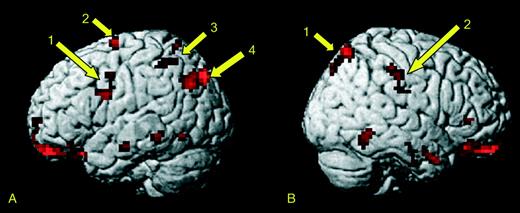
We predicted expertise effects in areas previously identified within the human mirror system, namely the premotor cortex, parietal cortex (intra-parietal sulcus, IPS), superior parietal lobe (SPL) and superior temporal sulcus (STS). Accordingly, we performed small volume corrections for multiple comparisons using 10 mm spheres centred on these areas, as follows: we used coordinates from Buccino et al. (2001) for premotor cortex, SPL and IPS, and from Grèzes et al. (2004) for STS. The results showed bilateral activation in premotor cortex corresponding to the expertise effect. We also found significant bilateral activations in the intraparietal cortex /sulcus and right superior parietal lobe (Fig. 2). Posterior parts of the STS were activated in the left hemisphere. Although we show bilateral activations in premotor and intraparietal cortex, the clusters for these activations were larger in the left hemisphere than in the right.
Effects of motor expertise on brain responses to action observation defined as the group by condition interaction. Projections of the activation foci on the surface of standard brain (Montreal Neurological Institute, MNI). Note that this projection renders onto the surface activity which may in fact be located in the sulci. Activations significant at P < 0.001 uncorrected are shown in red. For display purposes, an extent threshold of 10 voxels has been used. Arrows indicate predicted areas with activations significant at P < 0.05 after small volume correction using a 10 mm sphere. These are in the left hemisphere system (A), in (1) ventral premotor, (2) dorsal premotor, (3) IPS and (4) pSTS. In the right hemisphere (B) we show activations in (1) SPL and (2) IPS.
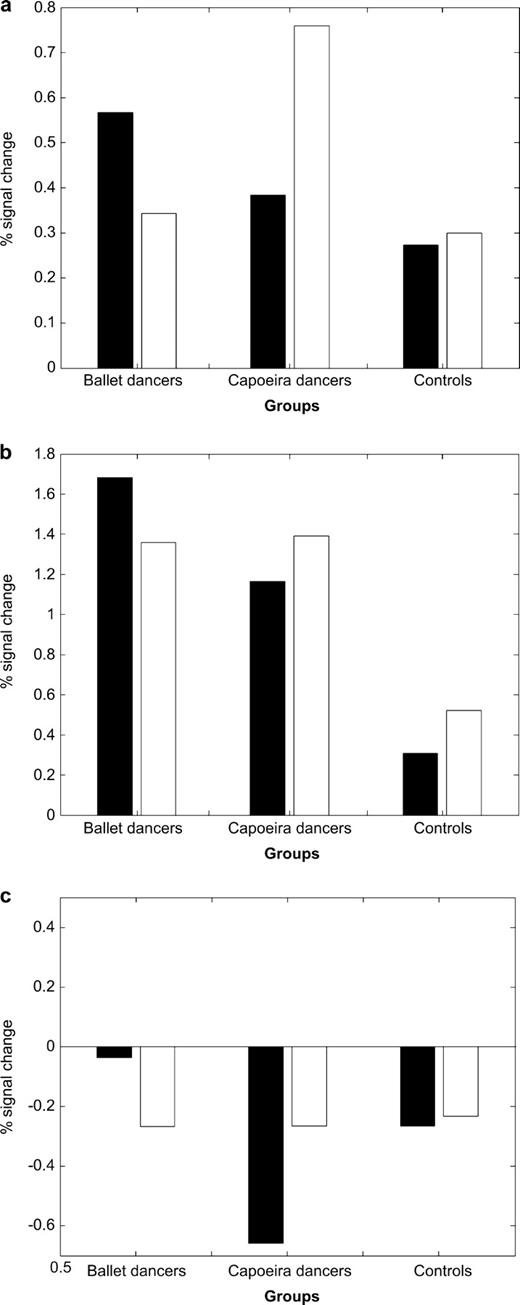
These significant interactions were further investigated by examining contrasts of parameter estimates (see Fig. 3). Experts had greater activation when observing the specific movement style that they could perform. This yielded a crossover pattern of interaction between group and stimulus type. Moreover, the same voxels in non-expert control subjects were essentially insensitive to stimulus type, confirming that the interaction depends on acquired motor skills, and not on visual properties of the stimuli. Finally, no significant activations corresponding to the main effects of expert group or stimulus type were found in mirror system areas.
Parameter estimates for the influence of motor expertise on action observation in the central voxels of areas classically identified with the human mirror system: (A) left precentral gyrus/dorsal premotor cortex (−24 −6 72), (B) left intra-parietal sulcus (−33 −45 54), (C) left posterior superior temporal sulcus (−39 −66 36). In all three areas, parameter estimates show that the effect of expertise is driven by a crossover interaction between the two groups of expert dancers and the two stimulus types. Stimulus type has minimal effects in control subjects. Black bars reflect parameter estimates for ballet stimulus and white bars reflect capoeira stimulus.
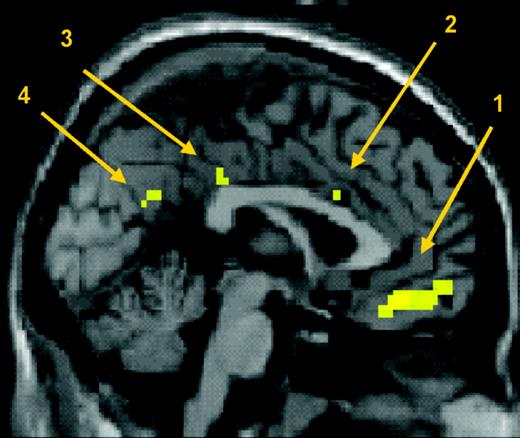
Beyond these predicted areas of interest, we also found other expertise effects which survived correction for multiple comparisons over the whole brain (P < 0.05). These included the medial frontopolar gyrus, the cingulate gyrus extending from its posterior part including the retrosplenial gyrus to its middle part and the right parahippocampal gyrus (see Fig. 4). Further activations are not discussed here since they were not specifically predicted, and did not survive correction. These are, however, listed in Table 1 for information.
Influence of motor expertise on brain responses to action observation: saggital section showing activation after correction for multiple comparisons across the whole brain at P < 0.05. (1) ventro-medial frontopolar gyrus, (2) cingulate gyrus, (3) posterior cingulate gyrus and (4) retrosplenial gyrus.
Discussion
Our results show that the brain's response to seeing an action is influenced by the acquired motor skills of the observer. Subjects showed stronger BOLD responses in classical mirror areas (Grèzes and Decety, 2001; Rizzolatti et al., 2001), including the premotor, parietal cortices and STS, when they observed dance actions that were in their personal motor repertoire than when they observed kinematically comparable dance actions that were not in their repertoire. Thus, expert ballet dancers showed greater activity in these areas when watching ballet moves than when watching capoeira moves, while expert capoeira dancers showed the opposite effect. Thus, while all groups saw the same stimuli, the mirror areas of their brains responded to the stimuli in a way that depended on the observer's specific motor expertise. This suggests that action observation may recruit such mirror areas to the extent that the observed action is represented in the subject's personal motor repertoire, i.e. if the subject has acquired the motor skills to perform such actions. Further evidence linking action observation to specific motor representations comes from the parameter estimates in our normal subjects. For these individuals, who had no motor experience of either ballet or capoeira, no such differences were detected. Taken as a whole, our results suggest that action observation in humans involves an internal motor simulation of the observed movement.
In addition, these results clarify what kind of motor representation is engaged by action observation. First, significant expertise effects suggest the mirror system codes complete action patterns, not just individual component movements. The dance styles studied here have quite similar components at the muscle level (both involve jumping, for example). Even though both groups of dancers could perform such primitive component movements, our stimuli evoked mirror system activity which varied with expertise. Previous studies emphasized homology between muscle groups in observation and execution (Fadiga et al., 1995; Buccino et al., 2001; Rizzolatti et al., 2001). Our results suggest that the mirror system is also sensitive to much more abstract levels of action organization, such as those that differentiate dance styles. To borrow a distinction from the motor control literature (Sanes and Donohue, 2000), our results show that the mirror system is concerned with observing skilled movements, not muscles. Second, we find that mirror system representations are linked to learned motor skills. A recent study of learning precisely timed patterns of finger movements (Sakai et al., 2002) reported premotor cortex activation associated with new learning of such patterns. These activations were over and above the effects of learning sequential order alone or temporal intervals alone. Those results suggest that premotor cortex may encode detailed action plans for complex movements. Our results suggest such action plans may also be activated by action observation.
The experiment's factorial design also excludes alternative interpretations of these effects. First, our result cannot be due to kinematic differences between ballet and capoeira stimuli, since we defined expertise as the interaction of a factorial design in which all groups of subjects saw both classes of stimuli. We also carefully matched kinematics across the dance two styles. Indeed, we found no main effect of stimulus type within the mirror system, and parameter estimates of control subjects showed similar activity in response to both kinds of stimuli. This suggests that kinematics differences do not contribute to our result. Second, our results are unlikely to reflect differences between groups in purely perceptual processing of the dance moves, since we found no evidence of expertise effect in brain areas classically associated with perceptual learning, such as the inferotemporal and occipital cortices (Gauthier et al., 2000). Movement expertise did modulate activity in middle temporal areas, perhaps reflecting semantic categorization (Vandenberghe et al., 1996) of the dance moves by experts but not by non-experts. However, this effect did not survive correction for multiple comparisons. Moreover, we suggest that any semantic categorization process would be parallel to and independent of the motor simulation conducted by the mirror system. Thus, Decety et al. (1997) showed that meaningful and meaningless actions differed only in the temporal and frontal activations evoked, while no differences were seen in the classical action observation system.
We have suggested that the increased BOLD responses in experts' mirror systems reflected their motor expertise. However, dance performers generally see more of their own dance style than of other dance styles. In particular, both classical ballet and capoeira involve extensive practice with other dancers. Could our results therefore reflect visual familiarity rather than motor expertise? We suggest three arguments against this possibility. First, the expertise effects we observed within the mirror system included areas classically considered as motor areas, such as left premotor cortex. Second, we saw no expertise effects in areas such as the fusiform gyrus, where effects of visual familiarity and perceptual learning have been repeatedly reported (Gauthier et al., 1999; Tarr and Gauthier, 2000). Finally, preliminary evidence from another study suggests that motor expertise has significant effects after effects of visual familiarity are controlled for (Glaser et al., 2004). In classical ballet, some moves are gender-specific, while others are performed by both women and men. Since dancers train together, all dancers are visually familiar with all moves. Female ballet dancers showed lower left parietal BOLD responses when watching male-only moves than when watching either female-only moves or moves performed in common by either male or female performers.
The expertise effect showed two distinct activations — one dorsal and one ventral — within the premotor cortex. The dorsal premotor activation was found bilaterally, though with a larger cluster size in the left hemisphere than in the right. Ventral premotor activation was seen in the left hemisphere only. Two distinct activations were also seen in the parietal cortex, bilateral activity in the intraparietal sulcus and superior parietal lobule in the right hemisphere only. Interestingly, we also found SPL activation in the left hemisphere, but this did not survive SVC using the coordinates of Buccino et al (2001). In general, this pattern of activations fits well with previous studies of somatotopic organization in premotor and parietal cortex. Our dance stimuli obviously involved the whole body, with large, proximal arm and leg movements. Primate studies suggest that movements of each body part are coded in independent, parallel parieto-frontal circuits that subserve somatotopically organized motor representations of the different effectors (Jeannerod et al., 1995; Rizzolatti et al., 1998). Thus, electrical stimulation of the monkey premotor cortex elicited complex postures (Graziano et al., 2002), with a dorsal-to-ventral somatotopic organization for leg and foot, arm and hand and finally face and mouth. A similar somatotopic organization for action observation was found in parietal and premotor cortex when human subjects watched a moving hand or a moving foot (Buccino et al., 2001). Our results are consistent this concept of direct somatotopic matching between visual stimuli of body parts and corresponding movements.
We also found a clear effect of expertise in a third element of the human mirror system, the left posterior STS. This region is functionally and anatomically distinct from other visual motion areas such as MT (Watson et al., 1993) and the kinetic occipital region (Van Oostende et al., 1997), because it does not respond well to coherent, translational motion or kinetic boundaries. Rather, the STS is involved in the perception of biological motion (Bonda et al., 1996; Grossman and Blake, 2002) and of hand, mouth and eye movements (Allison et al., 2000). As for the premotor and the parietal cortex, the present study shows an influence of motor expertise in a classically perceptual area such as the STS.
Finally, we identified a second set of areas influenced by expertise. This comprised the ventromedial frontal lobe, anterior/middle and posterior cingulate and parahippocampal gyrus. These areas did not form part of the initial hypotheses for the study. However, they survive statistical correction for multiple comparisons over the whole brain volume and are consistent with other findings relating these areas to emotional experience. The activation in the ventromedial frontal cortex recalls two previous theories of activation in this area. First, this area is routinely activated in emotion processing (see Steele and Lawrie, 2004, for a meta-analysis). In particular, it shows strong responses to pleasurable and rewarding stimuli (O'Doherty et al., 2003). Second, Decety and Chaminade (2003) have suggested that this area contributes to social judgement and the regulation of social behaviour. These two explanations are clearly not mutually exclusive in the context of expertise effects. Experts may show increased ventromedial frontal activation when watching their own movement style because it is particularly rewarding for them, or because they have a greater social engagement with the person they observe. Our study cannot distinguish between the emotional and the social-engagement aspects of this activation, and indeed was not designed to do so, though this would be a fruitful topic for future research.
The influence of expertise on cingulate, retrosplenial and parahippocampal activation is also consistent with these areas' role in episodic memory. Functional neuroimaging studies of retrieving items from memory have shown effects of familiarity on prefrontal activations, and also anterior and posterior cingulate (Burgess et al., 2001). The posterior activations may contribute to imagery and episodic recall from long-term storage of allocentric information maintained in other areas of the brain. The greater familiarity of experts with their own movement style may lead to stronger activation of brain mechanisms of episodic memory, even when watching another person.
The right parahippocampal region is involved in storing and maintenance of stimulus representations across long delays, and seems predominantly dedicated to visuospatial aspects of these processes (Maguire et al., 2003; Small et al., 2003). Moreover, the parahippocampal gyrus shows greater activity following when viewing meaningful as compared to meaningless actions (Decety et al., 1997). Actions that appear meaningless to inexpert subjects may appear more meaningful to experts, and additionally recruit circuits for semantic representation in the brain. The influence of expertise suggests that, taken together as a network, activation of these midline areas reflects a combination of episodic memory processes and the degree of engagement between the viewer and the stimuli during action observation.
Conclusion
In summary, we have shown a clear effect of acquired motor skills on brain activity during action observation. The network of motor areas involved in preparation and execution of action was also activated by observation of actions. Crucially this activation was stronger when the subjects had the specific motor representation for the action they observed. Therefore, the parietal and premotor cortex mirror system does not respond simply to visual kinematics of body movement, but transforms visual inputs into the specific motor capabilities of the observer. While all the subjects in our study saw the same actions, the mirror areas of their brains responded quite differently according to whether they could do the actions or not. We conclude that action observation evokes individual, acquired motor representations in the human mirror system. Our finding lends support to ‘simulation’ theories (Gallese and Goldman, 1998), according to which action perception involves covert motor activity (Jeannerod, 1994; Grèzes and Decety, 2001; Rizzolatti et al., 2001). This activation of motor representations through mere observation could have important applications in enhancing skill learning and in motor rehabilitation.
We are grateful to Deborah Bull and Emma Maguire (Royal Ballet), Tom Sapsford and Giuseppe Vitolo and Rodrigo Peres (Capoeira School, London), and Opher Donchin for advice and assistance. This work was supported by a Wellcome Trust Programme Grant and an EU Fifth Framework Program (R.P., J.G.), EU Marie Curie Research Training Site (B.C.), Leverhulme Trust Research Fellowship (P.H.) and MRC Co-operative Group Grant to the Institute of Cognitive Neuroscience (D.G.).
References
Allison T, Puce A, McCarthy G (
Baldissera F, Cavallari P, Craighero L, Fadiga L (
Berger SM, Carli LL, Hammersla KS, Karshmer JF, Sanchez ME (
Bonda E, Petrides M, Ostry D, Evans A (
Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ (
Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G (
Burgess N, Maguire EA, Spiers HJ, O'Keefe J (
Decety J, Grezes J (
Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F (
di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G (
Duvernoy HM (
Fadiga L, Fogassi L, Pavesi G, Rizzolatti G (
Gallese G, Goldman A (
Gallese V, Fadiga L, Fogassi L, Rizzolatti G (
Gallese V, Fogassi L, Fadiga L, Rizzolatti G (
Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC (
Gauthier I, Skudlarski P, Gore JC, Anderson AW (
Glaser DE, Calvo-Merino B, Grezes J, Passingham RE, Haggard P (
Grafton ST, Arbib MA, Fadiga L, Rizzolatti G (
Graziano MS, Taylor CS, Moore T, Cooke DF (
Grèzes J, Decety J (
Grèzes J, Armony JL, Rowe J, Passingham RE (
Grèzes J, Frith C, Passingham RE (
Grossman ED, Blake R (
Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (
Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Rizzolatti G (
Jeannerod M (
Jeannerod M, Arbib MA, Rizzolatti G, Sakata H (
Maguire EA, Valentine ER, Wilding JM, Kapur N (
O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ (
Perani D, Fazio F, Borghese NA, Tettamanti M, Ferrari S, Decety J, Gilardi MC (
Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F (
Rizzolatti G, Luppino G, Matelli M (
Rizzolatti G, Fogassi L, Gallese V (
Sakai K, Ramnani N, Passingham RE (
Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM (
Steele JD, Lawrie SM (
Stevens JA, Fonlupt P, Shiffrar M, Decety J (
Strafella AP, Paus T (
Tai YF, Scherfler C, Brooks DJ, Sawamoto N, Castiello U (
Tarr MJ, Gauthier I (
Van Oostende S, Sunaert S, Van Hecke P, Marchal G, Orban GA (
Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS (
Author notes
1Institute of Movement Neuroscience, University College London and Department of Basic Psychology, Faculty of Psychology, Universidad Complutense, Madrid, Spain, 2Institute of Cognitive Neuroscience and Department of Psychology, University College London, 17 Queen Square, London WC1N 3AR, UK, 3Laboratoire de Physiologie de la Perception et de l'Action, Centre National de la Reserche Scientifique-College de France, Paris, France and 4Wellcome Department of Cognitive Neurology and Functional Imaging Laboratory, Institute of Neurology, University College London and Department of Experimental Psychology, University of Oxford, Oxford, UK