-
PDF
- Split View
-
Views
-
Cite
Cite
Naama Barnea-Goraly, Vinod Menon, Mark Eckert, Leanne Tamm, Roland Bammer, Asya Karchemskiy, Christopher C. Dant, Allan L. Reiss, White Matter Development During Childhood and Adolescence: A Cross-sectional Diffusion Tensor Imaging Study, Cerebral Cortex, Volume 15, Issue 12, December 2005, Pages 1848–1854, https://doi.org/10.1093/cercor/bhi062
Close - Share Icon Share
Abstract
Maturation of brain white matter pathways is an important factor in cognitive, behavioral, emotional and motor development during childhood and adolescence. In this study, we investigate white matter maturation as reflected by changes in anisotropy and white matter density with age. Thirty-four children and adolescents aged 6–19 years received diffusion-weighted magnetic resonance imaging scans. Among these, 30 children and adolescents also received high-resolution T1-weighed anatomical scans. A linear regression model was used to correlate fractional anisotropy (FA) values with age on a voxel-by-voxel basis. Within the regions that showed significant FA changes with age, a post hoc analysis was performed to investigate white matter density changes. With increasing age, FA values increased in prefrontal regions, in the internal capsule as well as in basal ganglia and thalamic pathways, the ventral visual pathways, and the corpus callosum. The posterior limb of the internal capsule, intrathalamic connections, and the corpus callosum showed the most significant overlaps between white matter density and FA changes with age. This study demonstrates that during childhood and adolescence, white matter anisotropy changes in brain regions that are important for attention, motor skills, cognitive ability, and memory. This typical developmental trajectory may be altered in individuals with disorders of development, cognition and behavior.
Introduction
Childhood and adolescence is a period of dynamic behavioral, cognitive and emotional development. This development parallels significant changes in white matter structure (Yakovlev, 1967; Pfefferbaum et al., 1994; Reiss et al., 1996; Paus et al., 1999; Schmithorst et al., 2002). Thicker myelin sheathes, increased axonal diameter and improved organization of white matter tracts can improve signal transduction, and may be critical factors in allowing cognitive, behavioral, and emotional development to take place in an optimal fashion.
Different methodologies have been used to study white matter development. Postmortem histological studies have described an increase in white matter myelination throughout childhood and adolescence. However, these studies are limited by small sample sizes (Yakovlev, 1967; Benes et al., 1994). Magnetic resonance imaging (MRI) analyses of brain development in vivo have consistently reported increases in white matter volume and density from childhood through adolescence (Reiss et al., 1996; Giedd et al., 1999a,b; Paus et al., 1999). These changes are typically attributed to increases in axon diameter, myelination or concentration of iron, depending on the method of analysis (Reiss et al., 1996; Steen et al., 1997; Paus et al., 1999, 2001). However, debate continues as to which of these processes contribute to white matter changes throughout development (Paus et al., 1999; Schmithorst et al., 2002).
Structural neuroimaging investigations attempting to elucidate regional patterns of white matter development have yielded conflicting results. A large longitudinal study of children and adolescents revealed linear increases in white matter volume that were not region specific (Giedd et al., 1999a). In contrast, in a volumetric whole-brain MRI study, Reiss et al. reported prominent regional increases in frontal white matter volume during childhood (Reiss et al., 1996). Paus et al. showed age-related increases in white matter density in tracts constituting putative corticospinal or thalamocortical tracts, and in left fronto-temporal pathways, but not in frontal regions (Paus et al., 1999).
Recently, investigators have turned to diffusion tensor imaging (DTI) to further investigate the development of white matter, and to clarify the conflicting data from other structural imaging modalities. DTI uses the magnetic resonance signal to visualize water movement within axons, which can help characterize axonal structure. While several DTI studies have consistently shown increases in anisotropy indices throughout infancy and the first decade of life (Mukherjee et al., 2001; McGraw et al., 2002; Ulug, 2002; Miller et al., 2003), only two DTI studies investigated white matter development beyond childhood (Klingberg et al., 1999; Schmithorst et al., 2002). The first was a region-of-interest (ROI)-based study, in which seven children were compared to five adults. This study suggested that adults had greater white matter anisotropy than children in frontal regions (Klingberg et al., 1999). In the second study, Schmithorst et al. reported increased anisotropy with age in corticospinal tracts, the left arcuate fasciculus, and the right inferior longitudinal fasciculus in 33 subjects ranging in age from 5 to 18 years (Schmithorst et al., 2002). In this study, trace (a measure of overall diffusivity) values, but not anisotropy, were observed to increase with age in prefrontal regions.
Inconsistent prefrontal findings in studies using DTI are intriguing, particularly because there is significant maturation of higher-order executive functions during childhood and adolescence (Casey et al., 1997, 2000; Luna et al., 2001; Kwon et al., 2002; McGivern et al., 2002; Tamm et al., 2002). Frontal regions, with their extensive cortical and subcortical connections, are crucial for these functions (Cummings, 1993; Fuster, 2000).
In this study, we sought to further delineate the spatial development of white matter pathways in typically developing children and adolescents using DTI. Our measure of analysis was fractional anisotropy (FA), an intravoxel measure that yields values between 0 (isotropic diffusion, i.e. diffusion that is equal in all directions) and 1 (maximum anisotropic diffusion, i.e. the hypothetical case of diffusion along one axis) (Basser and Pierpaoli, 1996; Pierpaoli and Basser, 1996). The degree of diffusion anisotropy in a voxel is determined by microstructural features of the tissue in that particular image voxel, including fiber diameter and density, the myelin sheath and other oriented macromolecular structures that hinder proton diffusion, as well as macrostructural features such as intravoxel fiber-tract coherence (Pierpaoli and Basser, 1996). In addition, we conducted a voxel-based morphometry analysis, in which we examined age-related changes in white matter signal intensity, also referred to as ‘white matter density’ (Paus et al., 1999).
Based on cognitive and behavioral data as well as previous imaging studies, we anticipated age-related changes in white matter within prefrontal regions, corticospinal tracts and left fronto-temporal tracts. A second objective of our study was to better define which structural changes occur in white matter throughout development. To address this goal, we studied overlaps and discrepancies between fractional anisotropy (FA) and white matter density changes with age. It was hypothesized that this analysis would help to differentiate between structural changes in white matter that affect both FA and white matter density (e.g. increasing myelination and axonal density and diameter) (Barkovich et al., 1988; Pierpaoli and Basser, 1996; Paus et al., 1999), and other structural changes that affect only FA values (e.g. fiber orientation).
Materials and Methods
Subjects
Typically developing participants were recruited as control subjects through newspaper advertisements and parent networks. After providing a complete description of the study to participating subjects and their caretakers, written informed consent was obtained under protocols approved by the Institutional Review Board of Stanford University. All subjects were right-handed, in good health and without evidence of neurological or psychiatric disorder, as assessed utilizing a comprehensive telephone screening instrument and scores in the non-significant range on the Child Behavior Checklist (Achenbach, 1991). Each participant was administered intelligence testing using age-appropriate Wechsler Scales of Intelligence. Subjects with a Full Scale IQ (FSIQ) score <80 or >130 were excluded from the study, as we wished the sample to be representative of the general population. In all, 34 (18 male) typically developing children and adolescents participated in this study (mean age = 12.7 ± 3.3; age range = 6–19 years; mean FSIQ = 114 ± 9.5).
Image Acquisition
Each participant was scanned on a 1.5 T whole body GE Signa Horizon scanner (GE Medical Systems, Milwaukee), and received a three-dimensional, high-resolution T1-weighted anatomic gradient- and RF-spoiled rapid gradient echo sequence with the following pulse sequence: repetition time (TR) = 35 ms; echo time (TE) = 6 ms, flip angle (α) = 45°, number of excitations (NEX) = 1, field of view = 24 cm, matrix size = 256 × 256, for 124 contiguous slices of 1.5 mm width. Magnetic resonance signal excitation and reception was performed using a quadrature birdcage coil provided by vendor.
DTI image acquisition was accomplished with single-shot spin-echo echo-planar imaging (EPI) sequence with diffusion sensitizing gradients applied on either side of the 180° refocusing pulse (Moseley et al., 1991; Basser et al., 1994). Imaging parameters for the diffusion-weighted sequence were as follows: field of view (FOV) = 24 cm, matrix size 128 × 128, TE/TR = 106/6000 ms, 18 or 19 axial-oblique slices, slice thickness 5 mm/skip 1 mm, and fractional k-space acquisition. A spectral-spatial excitation RF pulse was used to select water protons only and served to avoid ghost artifacts from lipid-bound protons. The scan was prescribed from the top of the brain and included only the most superior part of the cerebellum. Diffusion gradient duration was δ = 32 ms, diffusion weighting was b = 900 s/mm2. In addition, two reference measurements (b0-scans) were performed and averaged for each slice with the diffusion-encoding gradients turned off.
Diffusion was measured along six non-collinear directions: xy, xz, yz, −xy, −xz and −yz. This pattern was repeated four times for each slice with the sign of all diffusion gradients inverted for odd repetitions.
Image Processing and Statistical Analyses
Eddy current effects in the diffusion-weighted images (i.e. geometric distortions that vary from one diffusion direction to the next) were unwarped prior to averaging (de Crespigny and Moseley, 1998). Averaging of the four magnitude images efficiently removed the effect of gradient cross-terms between the diffusion sensitizing and imaging gradients (Neeman et al., 1991). Fractional anisotropy (FA) was calculated for each voxel according to methods described by Basser and Pierpaoli (1996) to produce an FA image. A T2-weighted image map was used to determine normalizing parameters subsequently applied to the fractional anisotropy images using Statistic Parametric Mapping software (SPM99 software, Wellcome, UK). Normalized FA images were smoothed with a 4 mm kernel to increase the signal-to-noise ratio. Subsequently, a linear regression model was used to correlate the FA maps with subject's age on a voxel-by-voxel basis. Finally, to determine the presence of significant clusters of differences, the joint expected probability distribution of the height and extent of Z-scores with height (Z > 2.33; P < 0.01) and extent (Z > 1.67; P < 0.05) thresholds, was used to correct for spatial correlation in the data (Poline et al., 1997). Using a spatially normalized, average T1-weighted image (SPGR scans) for all subjects a white matter cerebral mask was created and used to highlight changes in white matter tracts eliminating noise and edge effects.
In the secondary analyses, we examined white matter density changes with age within regions that showed significant increases in FA values with age. The procedure and template used to spatially normalize the T1-weighted anatomic images were identical to those used to normalize the B0 images as described above. The T1-weighted images were normalized into standard stereotactic space using a combined 12-parameter affine and non-linear transformation (7 × 8 × 7 nonlinear basis functions). These data were then segmented to create images representing white matter, gray matter and cerebrospinal fluid. The segmented white matter images were then smoothed using a 4 mm kernel. Finally, within ROIs in which FA values were significantly correlated with age, a linear regression model was used to correlate the white matter density images with the subjects' ages using voxel-by-voxel unpaired t-tests (using SPM99). To identify significant voxel clusters and minimize Type II errors, both height (P < 0.01) and extent (P < 0.05) thresholds were employed. This analysis was performed with 30 of the original 34 subjects, as an SPGR was not acquired for four subjects.
Results
FA Changes with Age
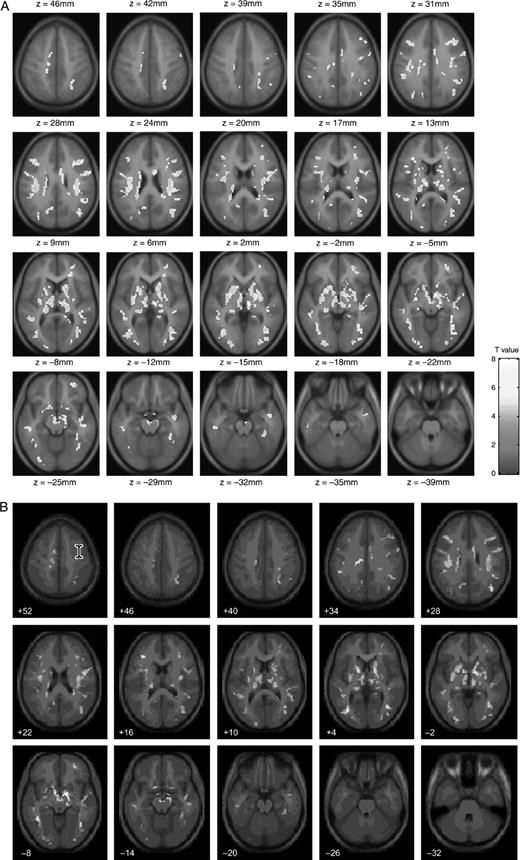
White matter showing increased FA values with age was seen in prefrontal regions, in the internal capsule, in pathways extending within the basal ganglia, and between the basal ganglia and the thalamus (Fig. 1A). Additional age-related increases in FA were observed in areas that appeared to correspond to cortico-thalamic and cortico-spinal tracts extending from sensory-motor regions, and in white matter of the ventral visual streams. Finally, age-related increases were observed in intrahemispheric tracts, which correspond to the location of the arcuate fasciculi extending from the frontal lobe to the superior and middle temporal gyrii and in the corpus callosum. No white matter regions were found to have significant decreases in FA values with age.
(A) Voxels that showed significant correlation between FA values and age (axial view). (B) Regions in which there was a significant increase in FA values with age (shown in red), and areas in which there was significant increase in both FA values and white matter density with age (shown in yellow). Areas in which the correlation between white matter density and age was more significant than correlation between FA values and age are shown in green.
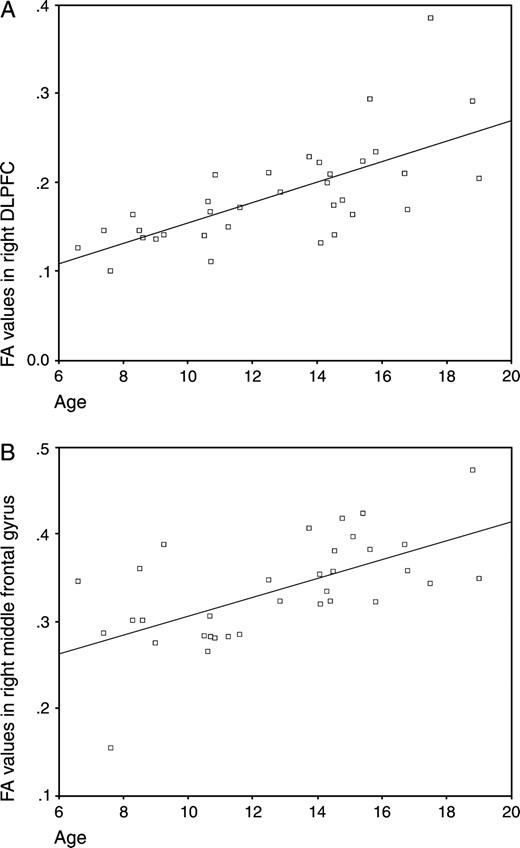
A correlation graph between FA values in sample brain regions and age. FA values were generated from two points of peak correlation between FA and age described in Table 1. (A) Right DLPFC; (B) right middle frontal gyrus.
Voxel coordinates in Talairach space and the associated Z-scores for the most significant voxel of each cluster in the statistical parametric map in which FA values were significantly correlated with age.
| Location of significant FA differences . | Cluster size in voxels . | Talairach coordinates of most significant voxel . | Z-score . |
|---|---|---|---|
| Basal ganglia, thalamus | 5762 | 8, −2, −10 | 5.94 |
| Sensory-motor strip | 188 | −4,−5,59 | 5.03 |
| Middle frontal gyrus adjacent to dorsolateral prefrontal cortex | 241 | −40,25,25 | 4.47 |
| Occipital pathway extending into the ventral visual pathway | 432 | −40,−72,7 | 4.46 |
| Occipital pathways extending into the parietal cortex and the ventral visual stream (adjacent to the middle and inferior occipital gyrus) | 795 | 38,−73,17 | 4.44 |
| Dorsolateral prefrontal cortex (DLPFC) | 249 | 46,23,26 | 4.40 |
| Superior temporal gyrus extending into the middle temporal gyrus. Around the superior temporal sulcus | 316 | −48,−33,3 | 4.32 |
| Left extrastriate regions | 222 | −22,−85,−3 | 3.98 |
| Adjacent to the interparietal sulcus bordering the angular gyrus | 156 | 28,−56,45 | 3.96 |
| Deep parietal white matter in the centrum semiovale | 172 | 28,−31,35 | 3.9 |
| Middle frontal gyrus | 126 | 32,30,15 | 3.89 |
| Precuneus medial parietal | 148 | −18,−65,25 | 3.81 |
| Right middle occipital gyrus | 127 | 28,−86,−2 | 3.8 |
| Inferior frontal gyrus extending into the orbitofrontal gyrus | 177 | 28,48,−6 | 3.74 |
| Location of significant FA differences . | Cluster size in voxels . | Talairach coordinates of most significant voxel . | Z-score . |
|---|---|---|---|
| Basal ganglia, thalamus | 5762 | 8, −2, −10 | 5.94 |
| Sensory-motor strip | 188 | −4,−5,59 | 5.03 |
| Middle frontal gyrus adjacent to dorsolateral prefrontal cortex | 241 | −40,25,25 | 4.47 |
| Occipital pathway extending into the ventral visual pathway | 432 | −40,−72,7 | 4.46 |
| Occipital pathways extending into the parietal cortex and the ventral visual stream (adjacent to the middle and inferior occipital gyrus) | 795 | 38,−73,17 | 4.44 |
| Dorsolateral prefrontal cortex (DLPFC) | 249 | 46,23,26 | 4.40 |
| Superior temporal gyrus extending into the middle temporal gyrus. Around the superior temporal sulcus | 316 | −48,−33,3 | 4.32 |
| Left extrastriate regions | 222 | −22,−85,−3 | 3.98 |
| Adjacent to the interparietal sulcus bordering the angular gyrus | 156 | 28,−56,45 | 3.96 |
| Deep parietal white matter in the centrum semiovale | 172 | 28,−31,35 | 3.9 |
| Middle frontal gyrus | 126 | 32,30,15 | 3.89 |
| Precuneus medial parietal | 148 | −18,−65,25 | 3.81 |
| Right middle occipital gyrus | 127 | 28,−86,−2 | 3.8 |
| Inferior frontal gyrus extending into the orbitofrontal gyrus | 177 | 28,48,−6 | 3.74 |
Voxel coordinates in Talairach space and the associated Z-scores for the most significant voxel of each cluster in the statistical parametric map in which FA values were significantly correlated with age.
| Location of significant FA differences . | Cluster size in voxels . | Talairach coordinates of most significant voxel . | Z-score . |
|---|---|---|---|
| Basal ganglia, thalamus | 5762 | 8, −2, −10 | 5.94 |
| Sensory-motor strip | 188 | −4,−5,59 | 5.03 |
| Middle frontal gyrus adjacent to dorsolateral prefrontal cortex | 241 | −40,25,25 | 4.47 |
| Occipital pathway extending into the ventral visual pathway | 432 | −40,−72,7 | 4.46 |
| Occipital pathways extending into the parietal cortex and the ventral visual stream (adjacent to the middle and inferior occipital gyrus) | 795 | 38,−73,17 | 4.44 |
| Dorsolateral prefrontal cortex (DLPFC) | 249 | 46,23,26 | 4.40 |
| Superior temporal gyrus extending into the middle temporal gyrus. Around the superior temporal sulcus | 316 | −48,−33,3 | 4.32 |
| Left extrastriate regions | 222 | −22,−85,−3 | 3.98 |
| Adjacent to the interparietal sulcus bordering the angular gyrus | 156 | 28,−56,45 | 3.96 |
| Deep parietal white matter in the centrum semiovale | 172 | 28,−31,35 | 3.9 |
| Middle frontal gyrus | 126 | 32,30,15 | 3.89 |
| Precuneus medial parietal | 148 | −18,−65,25 | 3.81 |
| Right middle occipital gyrus | 127 | 28,−86,−2 | 3.8 |
| Inferior frontal gyrus extending into the orbitofrontal gyrus | 177 | 28,48,−6 | 3.74 |
| Location of significant FA differences . | Cluster size in voxels . | Talairach coordinates of most significant voxel . | Z-score . |
|---|---|---|---|
| Basal ganglia, thalamus | 5762 | 8, −2, −10 | 5.94 |
| Sensory-motor strip | 188 | −4,−5,59 | 5.03 |
| Middle frontal gyrus adjacent to dorsolateral prefrontal cortex | 241 | −40,25,25 | 4.47 |
| Occipital pathway extending into the ventral visual pathway | 432 | −40,−72,7 | 4.46 |
| Occipital pathways extending into the parietal cortex and the ventral visual stream (adjacent to the middle and inferior occipital gyrus) | 795 | 38,−73,17 | 4.44 |
| Dorsolateral prefrontal cortex (DLPFC) | 249 | 46,23,26 | 4.40 |
| Superior temporal gyrus extending into the middle temporal gyrus. Around the superior temporal sulcus | 316 | −48,−33,3 | 4.32 |
| Left extrastriate regions | 222 | −22,−85,−3 | 3.98 |
| Adjacent to the interparietal sulcus bordering the angular gyrus | 156 | 28,−56,45 | 3.96 |
| Deep parietal white matter in the centrum semiovale | 172 | 28,−31,35 | 3.9 |
| Middle frontal gyrus | 126 | 32,30,15 | 3.89 |
| Precuneus medial parietal | 148 | −18,−65,25 | 3.81 |
| Right middle occipital gyrus | 127 | 28,−86,−2 | 3.8 |
| Inferior frontal gyrus extending into the orbitofrontal gyrus | 177 | 28,48,−6 | 3.74 |
White Matter Density Changes with Age
Within the regions of interest derived from the FA analysis results, there were prominent white matter density increases with age in the internal capsule, in inter-thalamic pathways, and in the corpus callosum. Additional white matter density increases with age were observed in prefrontal regions, in the arcuate fasciculus, and in visual pathways (Fig. 1B, in yellow).
Discussion
We found significant and widespread developmental changes in white matter anisotrophy (FA values) in typically developing children and adolescents between 6 and 19 years of age. Our findings are consistent with previous studies that have reported age-related increases in white matter anisotropy in the arcuate fasciculus, in motor areas, and in the internal capsule (Paus et al., 1999; Schmithorst et al., 2002). In addition, our study replicated, in a larger sample, age-related FA increases in prefrontal regions (Klingberg et al., 1999). Age-related changes in white matter FA values also were observed in four brain pathways that may be important for our understanding of changing brain function during development: the corpus callosum, white matter tracts within the basal ganglia, between the basal ganglia and the thalamus, and in ventral-visual pathways.
White Matter Anisotropy Changes with Age in Prefrontal Regions
As children mature, they become more capable of executing tasks that require complex cognitive functioning. Contributing to this cognitive development are capabilities such as working memory, inhibition and attention. These capabilities improve with age (Berman and Friedman, 1995; Luciana and Nelson, 1998; Bedard et al., 2002; Luna et al., 2001; Kwon et al., 2002), and have been attributed at least partially to prefrontal circuitry (Filley, 2002; Kwon et al., 2002; Konishi et al., 2003). Synaptic proliferation and pruning, as well as ongoing myelination, are assumed to be important mechanisms that shape cognitive development (Huttenlocher, 1979; Greenough et al., 1987; McGivern et al., 2002; Sowell et al., 2004). Supporting this assumption, there is evidence for gray matter loss, beginning around puberty, in sensorimotor areas that spreads during late adolescence into ‘higher-order’ cortical regions, including the dorsolateral prefrontal cortex (Gogtay et al., 2004). In another study, frontal cortical thinning was related to improved ability to retain and retrieve verbal and spatial information (Sowell ER et al., 2001).
Our data suggest that beyond the processes that occur in the prefrontal cortex, cognitive improvement with age may be due to more coherent, or more myelinated, non-cortical white matter circuitry in prefrontal regions.
This typical brain development may be altered in developmental disorders that cause impairments in working memory, attention or inhibition (e.g. attention deficit hyperactivity disorder, Obssesive-Compulsive disorder, Tourette's syndrome, and schizophrenia) (Weinberger et al., 1986; Park and Holzman, 1992; Purcell et al., 1998; Cohen et al., 1999; Merriam et al., 1999; Rucklidge and Tannock, 2002; Stevens et al., 2002; Channon et al., 2003). Interestingly, prefrontal white matter has been implicated in these disorders (Mac Master et al., 1999; Foong et al., 2001; Hoptman et al., 2002; Kates et al., 2002).
White Matter Anisotropy Changes with Age in the Corpus Callosum
The corpus callosum, the largest white matter tract in the brain, functions to connect left and right cerebral hemispheres. Callosal fibers are important for motor and sensory integration, attention, memory, and general cognitive functioning (Njiokiktjien et al., 1994; Brown et al., 1999; Eliassen et al., 1999; McDonald et al., 2001; Tomaiuolo et al., 2001; Bookstein et al., 2002; Dorion et al., 2002).
In our study, we found overlapping changes in FA and white matter density with age in the body of the corpus callosum, which contains fibers important for connecting motor, sensory and auditory cortices (Pandya, 1986). The observed increases in FA values and white matter density may be related to the improved motor skills during development.
White Matter Anisotropy Changes with Age in the Basal Ganglia, and Between the Basal Ganglia and Thalamus
Previous research has shown that the striatum undergoes structural changes during childhood and adolescence (Sowell et al., 1999). These changes are thought to result from normal dendritic pruning, myelination, and changes in iron deposition. Our study complements these findings and suggests that white matter tracts originating or terminating in the basal ganglia and thalamus also show temporal maturation during childhood and adolescence. The basal ganglia and thalamus have extensive reciprocal connections with the frontal cortex and the anterior cingulate (Graybiel, 2000). These pathways have an important regulatory influence on the cortex and are known to be involved in emotional, behavioral, cognitive, and motor functions as well as in attention, learning and memory (Cummings, 1993; Graybiel, 2000; Herrero et al., 2002; Packard and Knowlton, 2002).
The increases in anisotropy with age we observed in the basal ganglia, thalamus and prefrontal cortex may reflect maturation of these basal-ganglia-thalamo-cortical pathways. Accordingly, during normal development, improvement in attention, motor skills, cognitive ability and memory, as well as in the modulation of behavior, may be a result of white matter maturation and improved signal transmission in brain circuits connecting the prefrontal cortex and the basal ganglia.
White Matter Anisotropy Changes with age in Ventral Visual Pathways
Although the visual system matures early in development, the functions of the ventral visual pathways, such as the establishment of form, face, and object representations, may develop throughout life (Neville and Bavelier, 2002). Neuropsychological evidence also suggests that visual−spatial integration, another important function of the ventral visual pathways, develops during late childhood and adolescence (Kovacs et al., 1999; Kovacs, 2000). Our study provides the first anatomical evidence of white matter changes during development localized to the ventral visual pathways that may correspond with this cognitive trajectory.
FA Changes versus White Matter Density Changes with Age
The underlying histological processes that lead to increases in white matter anisotropy with age are currently unknown. Changes in myelination, axonal diameter and density presumably affect both white matter density and anisotropy values. In contrast, changes in fiber orientation would probably affect white matter anisotropy but not white matter density. In this study, some regions showed overlap between anisotropy and density changes with age, while others had FA changes that were independent of the white matter density changes. This finding suggests that there are different histological processes acting in concert during white matter development.
Although white matter density changes with age corresponded, to some degree, with most major pathways that showed FA increases with age, the most significant overlap was observed in the internal capsule and in regions that correspond to pathways within the thalamus. This finding may reflect the fact that brain pathways are tightly packed in these areas, so that even small changes with age in myelin and fiber tract density result in a higher signal change for both FA and white matter density. Alternatively, this finding may reflect regional increases in axonal myelination, axonal diameter, or fiber tract density, or a combination of these processes. A caveat should be mentioned regarding regions of overlap that were observed in white matter bordering gray matter, as these findings may reflect subtle discrepancies in spatial normalization.
Many regions in which FA values increased with age did not show concurrent increases in white matter density (see Fig. 1B, red areas). This discrepancy may reflect a true difference between white matter anisotropy and white matter density changes with age. However, interpretation should be made with caution as regional differences in statistical power between the two methods may underlie some or all of these findings.
Interestingly, most areas in which FA values increased, but white matter density did not, tended to be in the periphery of the tracts, in regions that are closest to the gray matter/white matter borders. This could be explained by a neurodevelopmental sequence for which the peripheral changes in FA white matter values arise from fine-tuning of the direction and increased coherence of white matter tracts in association with brain maturation. Specifically, these data suggest that as neuronal connections are strengthened and pruned during development (Huttenlocher and Dabholkar, 1997), axons become more organized and coherent, resulting in increased anisotropy in those regions.
Future Directions and Conclusion
Future studies are needed to extend the findings presented here. First, while our study utilized a relatively large sample size, it also was cross-sectional. Longitudinal designs with even larger sample sizes will provide more accurate information as to the nature of white matter development in childhood and adolescence. In addition, future studies using new fiber tracking techniques in DTI will be useful in delineating the specific fiber tracts that show a significant change in FA values with age. Specifically, it would be important to investigate whether the basal ganglia, thalamic, and prefrontal pathways that showed FA maturation with age are continuous. Finally, further studies are needed to assess whether the observed white matter maturation is associated with changes in function.
In conclusion, this study provides new evidence of spatial and temporal development of white matter throughout childhood and adolescence. Our findings also suggest that particular histological phenomena underlie this development on a regional basis. Studies such as these will be helpful in eventually delineating how typical developmental trajectory is altered in individuals with disorders of development, cognition, and behavior.
The research presented in this manuscript was supported by NIH grant HD031715, HD40761 and the Sinclair Training Neuroimaging Fund.
References
Achenbach T (
Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D (
Basser PJ, Pierpaoli C (
Basser PJ, Mattiello J, LeBihan D (
Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R (
Benes FM, Turtle M, Khan Y, Farol P (
Berman S, Friedman D (
Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM (
Brown WS, Jeeves MA, Dietrich R, Burnison DS (
Casey BJ, Trainor RJ, Orendi JL (
Casey BJ, Giedd JN, Thomas KM (
Channon S, Pratt P, Robertson MM (
Cohen JD, Barch DM, Carter C, Servan-Schreiber D (
de Crespigny AJ, Moseley ME (
Dorion AA, Sarazin M, Hasboun D, Hahn-Barma V, Dubois B, Zouaoui A, Marsault C, Duyme M (
Eliassen JC, Baynes K, Gazzaniga MS (
Foong J, Symms MR, Barker GJ, Maier M, Woermann FG, Miller DH, Ron MA (
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (
Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX (
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (
Herrero MT, Barcia C, Navarro JM (
Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO (
Huttenlocher PR (
Huttenlocher PR, Dabholkar AS (
Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, Kofman O, Singer HS, Denckla MB, Pearlson GD, Kaufmann WE (
Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M (
Konishi S, Jimura K, Asari T, Miyashita Y (
Kovacs I, Kozma P, Feher A, Benedek G (
Kwon H, Reiss AL, Menon V (
Luciana M, Nelson CA (
Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA (
Mac Master FP, Keshavan MS, Dick EL, Rosenberg DR (
McDonald CR, Crosson B, Valenstein E, Bowers D (
McGivern RF, Andersen J, Byrd D, Mutter KL, Reilly J (
McGraw P, Liang L, Provenzale JM (
Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA (
Miller JH, McKinstry RC, Philip JV, Mukherjee P, Neil JJ (
Moseley ME, Wendland MF, Kucharczyk J (
Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC (
Neeman M, Freyer JP, Sillerud LO (
Neville H, Bavelier D (
Njiokiktjien C, de Sonneville L, Vaal J (
Packard MG, Knowlton BJ (
Park S, Holzman PS (
Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A (
Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC (
Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO (
Pierpaoli C, Basser PJ (
Poline JB, Worsley KJ, Evans AC, Friston KJ (
Purcell R, Maruff P, Kyrios M, Pantelis C (
Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla Mbyte (
Rucklidge JJ, Tannock R (
Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK (
Sowell ER, Delis D, Stiles J, Jernigan TL (
Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW (
Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW (
Steen RG, Ogg RJ, Reddick WE, Kingsley PB (
Stevens J, Quittner AL, Zuckerman JB, Moore S (
Tamm L, Menon V, Reiss AL (
Tomaiuolo F, Nocentini U, Grammaldo L, Caltagirone C (
Ulug AM (
Weinberger DR, Berman KF, Zec RF (
Author notes
1Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA, 2Program in Neuroscience, Stanford University, Stanford, CA 94305, USA, 3Neuroscience Institute, Stanford University, Stanford, CA 94305, USA, 4Department of Psychology, Children's Hospital of Orange County, Orange, CA 92868, USA and 5Lucas MRS/I Center Department of Radiology, Stanford University, Stanford, CA 94305, USA