-
PDF
- Split View
-
Views
-
Cite
Cite
Roberto Toro, Yves Burnod, A Morphogenetic Model for the Development of Cortical Convolutions, Cerebral Cortex, Volume 15, Issue 12, December 2005, Pages 1900–1913, https://doi.org/10.1093/cercor/bhi068
Close - Share Icon Share
Abstract
The convolutions of the mammalian cortex are one of its most intriguing characteristics. Their pattern is very distinctive for different species, and there seems to be a remarkable relationship between convolutions and the architectonic and functional regionalization of the cerebral cortex. Yet the mechanisms behind the development of convolutions and their association with the cortical regionalization are poorly understood. Here we propose a morphogenetic model for the development of cortical convolutions based on the structure of the cortex as a closed surface with glial and axonal fibres pulling radially, the fundamental mechanical properties of cortex and fibres (elasticity and plasticity), and the growth of the cortical surface. The computer simulations of this model suggest that convolutions are a natural consequence of cortical growth. The model reproduces several aspects of convolutional development, such as the relationship between cortical surface and brain volume among mammals, the period of compensation in the degree of convolution observed in gyrencephalic brains and the dependence of the degree of convolution on cortical thickness. We have also studied the effect of early cortical regionalization on the development of convolutions by introducing geometric, mechanic and growth asymmetries in the model. The morphogenetic model is thus able to reproduce the gradients in the degree of convolution, the development of primary, secondary and tertiary convolution, and the overproduction of sulci observed in animals with altered afferent cortical connections.
Introduction
The structure of the cerebral cortex is strongly conserved in the radial direction throughout the mammalian species. However, in the tangential direction it is one of the most variable and distinctive parts of the nervous system. The architectonic, connectional and functional specialization of the cortical surface seems to be closely related to the pattern of convolutions in gyrencephalic mammals (Connolly, 1950; Woolsey, 1960; Welker and Campos, 1963; Welker, 1990). Although in recent years important steps have been made towards understanding the genetic control of cortical regionalization (for reviews, see Pallas, 2001; Ragsdale and Grove, 2001; O'Leary and Nakagawa, 2002; Job and Tan, 2003; Krubitzer and Kahn, 2003), the processes underlying the development of cortical convolutions and their relationship to cortical regionalization remain essentially unknown.
In this article we propose a simple morphogenetic model to study the fundamental mechanisms of cortical folding. It is based on (i) the basic structure of the cortex as a closed surface with glial and axonal fibres pulling radially; (ii) the fundamental mechanical properties of cortex and fibres (elasticity and plasticity); and (iii) the growth of the cerebral cortex. The model suggests how the mechanical properties of the developing brain, along with the early architectonic regionalization of the cerebral cortex, lead to the development of convolutions and to their distribution. It provides a theoretical basis to combine the two principal theories of cortical folding: the mechanical theories of gyrification (Richman et al., 1975; Todd, 1982) and the genetic theories of gyrogenesis (Welker, 1990; Van Essen, 1997).
We begin by briefly reviewing the main stages of gyrification and the recent experimental evidence concerning the mechanisms of convolution development. [The literature has been reviewed in depth by Welker (1990).] Then we introduce our morphogenetic model as well as a finite-elements implementation with which the development of cortical convolutions in the model can be studied dynamically. Three processes by which the early regionalization of the cerebral cortex could affect the development and distribution of cortical folding are considered: (i) the effect of asymmetries in the geometry of the model; (ii) the effect of asymmetries in its mechanical properties; and (iii) the effect of asymmetries in its growth. Finally, we discuss the theoretical implications of our model in order to understand the phenomena of the morphogenesis of cortical convolutions.
Development of Cortical Convolutions
At the end of cell migration the cerebral hemispheres are smooth and undifferentiated. This is when many fundamental processes of cortical development begin, including arrival of afferents, neuronal growth and differentiation, axogenesis, dendrogenesis, synaptogenesis, glial cell proliferation and cortical lamination. In most of the mammals with large brains this period of rapid growth coincides with the emergence of cortical convolutions (Welker, 1990). The first convolutions to develop, the so-called primary convolutions, are more or less invariant in their location and configuration. Primary convolutions, such as the calcarine and the central sulcus, begin to develop at midgestation. These convolutions are soon followed by secondary and then tertiary convolutions, which are progressively less deep, more numerous and variable. In the human brain, tertiary convolutions continue to develop after birth, throughout the first year of life (Chi et al., 1977; Ono et al., 1990; Armstrong et al., 1995; Garel et al., 2001).
The mechanisms underlying the formation and distribution of cortical foldings are still poorly understood. A frequent hypothesis is that convolutions are the effect of cerebral growth within the limits of the cranial volume (Le Gros Clark, 1945). Although brain growth seems indeed to be important, many experiments show that cranial volume alone is not a determinant factor (Barron, 1950; Goldman and Galkin, 1978; Rakic, 1988; Dehay et al., 1996; Haydar et al., 1999; Chenn and Walsh, 2002; Kingsbury et al., 2003). The role of the available volume was directly studied by Barron (1950): despite the extraction of large quantities of cortical and subcortical tissue from sheep foetuses, at full term the remaining brain had developed cortical convolutions of almost normal size and configuration. On the other hand, the increased cortical growth in mutant mice due to depressing programmed cell death (Haydar et al., 1999; Kingsbury et al., 2003) or expanding the precursor pool during neurogenesis (Chenn and Walsh, 2002, 2003) resulted in an enlarged forebrain, an enlarged cranium and even the development of gyri and sulci resembling those of higher mammals.
While cranial volume is not essential for cortical folding, other mechanical factors seem to determine some aspects of the distribution of convolutions (Le Gros Clark, 1945; Richman et al., 1975; Todd, 1982). Connolly (1950) suggested that sulci can have a mechanical influence onto their neighbouring sulci, which he called compensation, i.e. a long period where short or shallow sulci can develop beside particularly long or deep sulci. This has been interpreted as a mechanism for the maintenance of a stable degree of folding in late brain development (Armstrong et al., 1995). A similar mechanism of influence has been proposed for the corpus callosum (Le Gros Clark, 1945; Malamud and Hirano, 1974; Ono et al., 1990). The corpus callosum is one of the first structures to develop in the human brain, and its congenital absence is linked to the disruption of the whole sulcal pattern on its medial surface, including the absence of a parallel cingulate gyrus and of a radial pattern of sulci. Yet, mechanical factors alone seem to be insufficient to explain the regularities in the distribution of cortical convolutions. For example, in humans the highly variable pattern of gyrification (Ono et al., 1990) shows a strong correlation between monozygotic twins (Biondi et al., 1998; White et al., 2002) which could hardly be explained solely by mechanical constraints.
In fact, classical studies of the areal organization of the cerebral cortex have shown a remarkable relationship between the pattern of convolutions and, for example, the cytoarchitectonic regions of the cortical surface (Brodmann, 1909; Elliot Smith, 1931), which has lead some authors to suggest that the development and distribution of convolutions are a direct result of cytoarchitectonic differentiation (Connolly, 1950; Welker, 1990). Many sulci have been described which separate different cytoarchitectonic areas. Examples of these limiting sulci are the central sulcus, which separates the primary motor cortex from the primary somatosensory cortex, and the lunate sulcus, which separates the striate cortex from the extrastriate cortex in widely separated species of primates (Connolly, 1950; Welker, 1990). Other sulci have been described which divide a single architectonic region. An example of these axial sulci is the calcarine sulcus, which separates the upper and lower halves of the primary visual cortex. However, the relationship between convolutions and cytoarchitecture seems to be more complex than a simple correspondence of sulcal fundi and cytoarchitectonic borders. In various primates cortical areas appear to shift position with respect to sulci (Connolly, 1950), and in the human cortex cytoarchitectonic borders seem to be only approximately related to sulcal fundi (Rademacher et al., 2001). Even while recent studies on human brain mapping may suggest a dissociation between cytoarchitecture and convolutional anatomy (Zilles et al., 1997; Roland and Zilles, 1998; Geyer et al., 1999; Amunts et al., 1999; Hasnain et al., 2001; Rademacher et al., 2001), it seems clear that a genetic factor plays a central role in the definition of the cortical folding pattern (Connolly, 1950; Welker, 1990; Rakic, 2004).
During ontogenesis the development of convolutions is related to the processes of cortical development and to the mechanisms underlying cortical specification. Many pathologies, such as microgyria, polymicrogyria, pachygiria and lissencephaly, appear to be associated with a remarkably abnormal cortical layering (Malamud and Hirano, 1974; Richman et al., 1975; Escourolle and Poirier, 1977; Piao et al., 2004). For example, microgyric brains present a profusely folded cortical surface, with abnormally small gyri and sulci, and where most layer V cells are absent. On the other hand, lissencephalic cortices have a smooth surface and extremely reduced layers II and IV. Indeed, Richman et al. (1975) have proposed a mechanical model where convolutions are produced by a differential growth of inner and outer cortical layers.
The disruption of the development of cortical afferents is also associated with important modifications of gyrification (Goldman and Galkin, 1978; Goldman-Rakic, 1980; Rakic, 1988; Dehay et al., 1996). Early unilateral or bilateral resection of the prospective prefrontal cortex in macaque foetuses produces symmetric ectopic sulci in the cerebral hemispheres. These sulci are not only restricted to the regions surrounding the lesion, but also develop in the temporal and in the occipital lobes, which suggests a relationship between development of cortical convolutions and of corticocortical connectivity (Goldman-Rakic, 1980). Further evidence for this relationship comes from a series of experiments of enucleation of macaque foetuses (Rakic, 1988, 1991; Dehay et al., 1989, 1991, 1996). These experiments show that the early bilateral enucleation produces an impressive overproduction of sulci in the occipital lobes as well as a large cytoarchitectonic reorganization of the presumptive primary visual cortex. In the enucleates the occipital lobes develop a symmetric pattern of convolutions up to four times more numerous than in the normal macaque. The small dimples of the extrastriate cortex in the normal animal become fully developed sulci in the enucleate. At birth the striate cortex of enucleates is up to 70% smaller than in the normal macaque whereas the total cortical surface remains unchanged (Dehay et al., 1996). The region normally related to the striate cortex develops a cytoarchitecture almost indistinguishable from that of extrastriate cortex (Dehay et al., 1996), and patches with a novel cytoarchitecture, termed area X, have been reported (Rakic, 1988, 1991). Indeed, thalamocortical afferents seem to play an important role in the definition and maintenance of cytoarchitectonic areas (Dehay et al., 2001; Pallas, 2001; O'Leary and Nakagawa, 2002; López-Bendito and Molnár, 2003).
Morphogenetic Model for the Development of Cortical Convolutions
Many different factors participate in the definition of the adult shape and pattern of the cortical convolutions (Welker, 1990). We propose a morphogenetic model to study the basic mechanisms of cortical folding where only the main traits of the structure of the developing brain are considered. A detailed account of the shape of convolutions in adult animals is then beyond the scope of our model.
The developing brain is modelled as a growing closed surface, representing the cerebral cortex, pulled radially by fibres, representing radial glia and early axonal connectivity. Two fundamental mechanical properties are considered in the model of the cerebral cortex and fibres: its elasticity, which allows a recovery of the initial shape following deformation, and its plasticity, which allows a permanent modification of the shape if a strong or continuous force is applied. Together, these two properties are referred to as elastoplasticity.
The radial glial fibres (Rakic, 1971, 2003) and the early axonal connections of the developing brain are modelled as elastoplastic radial elements which attach the growing cerebral cortex to the centre. The mechanical properties of these radial elements represent the elasticity of radial glia and axonal fibres, and their plastic elongation under tension (Chada et al., 1997; Van Essen, 1997). The orientation of the radial elements corresponds to the orientation of radial glia during development and with the early orientation of multiple projection systems. The maturation of the nervous system induces a deformation of the original radial organization, even so, many projection systems in the adult human brain, such as the corona radiata, conserve a remarkable degree of radial organization.
The cerebral cortex is modelled as a closed elastoplastic layer, which we call the cortical layer. The elastoplasticity of the cortical layer represents the mechanical properties of the cortical tissue, its cells (Zhu et al., 2000) and neuropil (Chada et al., 1997). The growth of the cortical layer in the model represents the total effect of the many concomitant processes of cortical development: growth and development of neurons, proliferation of glial cells, development of synapses, etc. The early regionalization of the cerebral cortex is represented by anisotropies in the characteristics of the cortical layer, e.g. gradual or abrupt changes in its mechanical properties, or in its growth rate. Recent experimental evidence shows that the developing cerebral cortex present gradients of signalling molecules and discrete regions of gene expression that seem to precede cortical regionalization in the adult. The specification and differentiation of the neocortex appears to result from the interaction between intrinsic genetic regulation and extrinsic influences, such as those mediated by thalamocortical afferents (Rakic, 1988; O'Leary, 1989). Signalling molecules diffused by a discrete number of patterning centres induce gradients of regulatory genes in the ventricular zone. These gradients, transposed to the cortical plate by radially migratory neurons, regulate the development of discrete areas and of the thalamocortical and corticocortical connectivity (Rakic, 1988; Levitt et al., 1997; Miyashita-Lin et al., 1999; Nakagawa et al., 1999; Bishop et al., 2000; Fukuchi-Shimogori and Grove, 2001, 2003; Garel et al., 2003). Together, intrinsic and extrinsic factors control the growth and differentiation of cortical tissue, which provides mechanical anisotropies from the earliest stages of cortical development.
The morphogenetic model does not contain any mechanism explicitly devised for the convolutions to develop, such as an increased growth rate of the pro-gyral cortex (Smart and McSherry, 1986a,b; Welker, 1990), differential tension of the white matter fibres on the pro-sulcal cortex (Dehay et al., 1996; Van Essen, 1997; J. Regis, J. Mangin, T. Ochiai, V. Frouin, D. Riviere, A. Cachia, I. Do, Y. Samson, unpublished manuscript), or differential growth of the inner and outer strata of the cortex (Richman et al., 1975). The results that we present in the following sections suggest that the morphogenesis of convolutions is a natural consequence of cortical growth, and that the early anisotropies of the cortical tissue can induce, modulate and guide the development of the folding pattern.
Methods
We construct a finite-elements implementation of the morphogenetic model so as to study the dynamics of cortical folding. For simplicity, the model is implemented in two dimensions. In the initial configuration the cortical layer is a circular structure composed of quadrilateral elements of width qw and thickness qh. The quadrilateral elements are attached to the centre of the model by linear elements that represent the radial elements of the morphogenetic model (Fig. 1). In this section we introduce first the equations that control the elastoplasticity of the radial elements, and then the equations for elastoplasticity and growth of the cortical layer. In the next section we present the results of the simulation of the morphogenetic model.
Finite-elements model of the developing cerebral hemisphere. (A) Initial shape of the finite-elements model. The cortical layer is composed of continuous quadrilateral elements attached to the centre by linear radial elements. (B) Dimensions of the finite-elements model. The initial length of the radial elements is rf0, the initial size of the quadrilateral elements of the cortical layer is qh in the radial direction and qw in the tangential direction. (C) Calculation of the deformation forces in the quadrilateral elements. The forces in each quadrilateral element are calculated as the difference between the actual deformed configuration and the rest configuration of the quadrilateral translated in tdr and rotated in θdr. Coordinates of the rest configuration:
Mechanically, the behaviour of each individual element of the cortical layer and of the radial elements is described by two equations: an equation for its elasticity and another for its plasticity.
Radial Elements
Cortical Layer
The elastic forces for each quadrilateral element of the cortical layer are obtained from the difference between the actual deformed configuration and its rest configuration. The deformed and reference configurations are defined by the coordinates of its corners, ci and
The elastic constant kc determines both the tangential elasticity of the cortical layer and its rigidity (which defines the force necessary to bend it). In the finite-elements model, bending the cortical layer deforms its quadrilateral elements: their external side is in tension while their internal side is in compression. The bending force necessary to produce the same curvature in different cortical layers depends on the thickness qh of their quadrilateral elements.
This produces a growth function with a typical sigmoidal shape. The logistic-growth function has been used to model many growth processes concerning brain development (Armstrong et al., 1995).
The displacement of each point on the cortical layer is given by the addition of the forces exerted by the corresponding radial element and the two quadrilateral elements which share that point. In the current implementation of the morphogenetic model no detection of mechanical contact is performed. This limits the level of growth that we can set for the cortical layer in order to avoid self-intersections (the C source code and an objective-C implementation of the morphogenetic model are available at http://www.snv.jussieu.fr/insermu483/geometricatlas/).
Results
Development of Convolutions
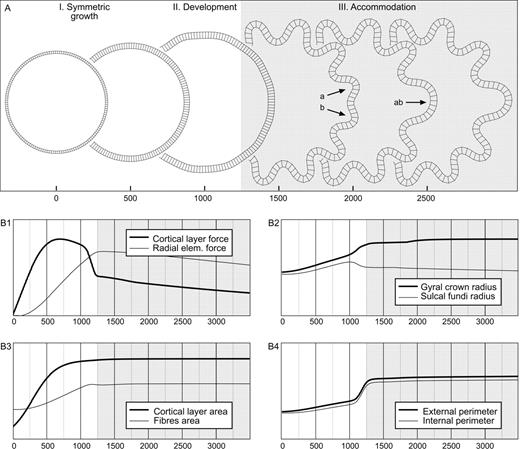
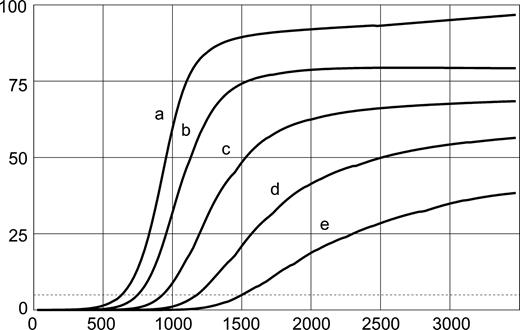
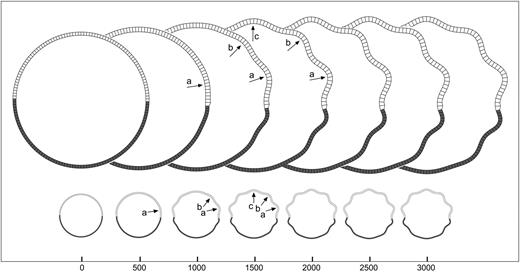
Convolutional development in the morphogenetic model is illustrated in Figure 2. These convolutions are produced only by the growth of the cortical layer. The initial geometry of the model is circular, and the growth parameters K, m, the elastic parameters kc, kf and the plastic parameters τc, τf are constant. Figure 2A shows snapshots of the model at intervals of 500 iterations, and Figure 2B shows the curves for the total force (Fig. 2B1), total radius (Fig. 2B2), total area (Fig. 2B3) and total perimeter (Fig. 2B4) of the model throughout the simulation.
Development of convolutions in the model. (A) Screenshots of the model at 500 iteration steps (radial elements are not drawn to avoid burden). Initially the model expands symmetrically without developing convolutions, then convolutions develop, and finally the convolutions are accommodated in the cortical layer. In the accommodation stage, convolutions can eventually fuse, as can be seen at the right side of the model, where convolutions a and b become a single convolution ab. (B) Evolution of the force, total radius, total area and total perimeter of the model. The curves represent 3000 iterations of the simulation. During the stage of symmetric growth the forces and the total area augment rapidly, with a slight augmentation of the perimeter and the radius. The development of convolutions is accompanied by a marked decrease in the forces and an augmentation of the perimeter. Elements = 150, qh = 4.5, qw = 4, kc = 0.5, τc = 4 × 104, kf = 0.05, τf = 104, K = 100, m = 5 × 10−3.
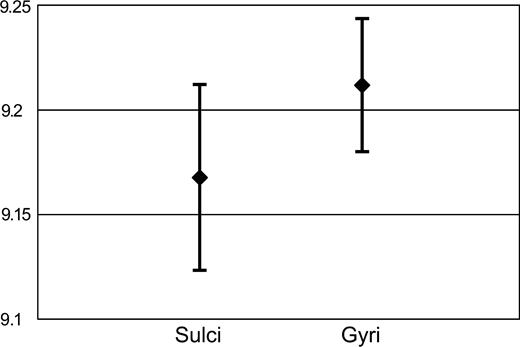
We distinguish three main stages in the development of convolutions in the model:At the end of the simulation, the position of sulcal fundi is almost the same that at the beginning (Fig. 2B2, light line). Furthermore, gyri are significantly thicker than sulci (Fig. 3).
Symmetric growth. In this first stage (iterations 0–750), the growth of the cortical layer produces a rapid increase of the total force in the cortical layer and radial elements (Fig. 2B1), a slow increase in its perimeter (Fig. 2B4) and a faster quadratic increase in the area of the radial elements' region (Fig. 2B3, light line). The growth of the area of the cortical layer, controlled by the logistic-growth function, is shown in the Figure 2B3 (heavy line).
Development of convolutions. In this second stage (iterations 750–1250), folds of a similar tangential size and depth develop. This moment is characterized by the rapid displacement of future gyri to the outside (Fig. 2B2, heavy line), as well as by a marked decrease in the total force just after folding. In fact, the total force in the convoluted model is similar to that at the beginning of the simulation, but for almost twice the perimeter.
Accommodation of convolutions. In this third stage (beginning at iteration 1250), the newly developed convolutions are accommodated in the available perimeter and eventually fuse. Figure 2A shows an example of such an accommodation. As the size of convolutions depends mainly on the thickness and mechanical properties of the cortical layer and not on its total perimeter, convolutions can develop that do not have enough space to be completely deployed in the available cortical perimeter. Soon after folding, two of these ‘truncated’ convolutions appear at the left side of the model in Figure 2A (arrows a and b, iteration 1500). In subsequent iterations they fuse to form a composed convolution at the right side (arrow ab, iteration 2000), which decreases the total number of convolutions from 13 to 12. Without the plastic component of the model, this stage of accommodation could last indeterminately. The adaptation of the radial elements and the cortical layer to deformations of long duration ensures the stability of the model.
Gyral and sulcal thickness. Mean percentual thickness of the cortical layer for gyri and sulci. Gyral and sulcal thickness have been evaluated in the model after 2500 iterations. Thickness is reported as a percent of the mean radius of the cortical layer. The cortical layer in gyri is significantly thicker than in sulci (p = 0.0075). Error bars represent SD.
Cortical Surface and Brain Volume
One striking characteristic of the mammalian brain is the relationship observed between cortical surface and the total brain volume through evolution. If the brains of different species were just scaled versions of one another, the cortical surface should vary to two-thirds the power of the brain volume. However, allometric studies of this relationship show that in gyrencephalic brains the cortical surface increases almost linearly with brain volume (Jerison, 1973, 1982; Prothero and Sundsten, 1984; Hofman, 1991; Prothero, 1997; Changizi, 2001). Hence, during brain growth, gyrification allows for an increasingly large cortical surface.
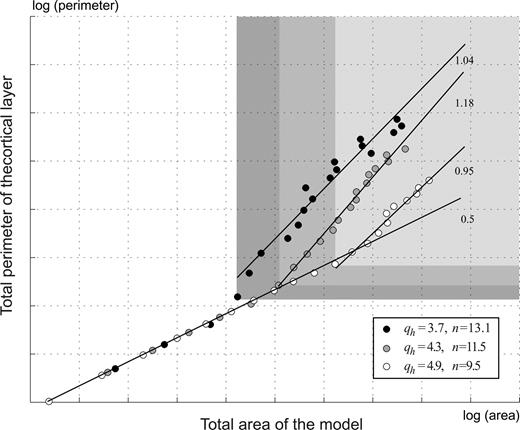
We have studied this relationship by analysing the variation of the perimeter of the cortical layer as a function of the total area of the model. In our analysis the cortical layer's perimeter represents the cortical surface area, and the total brain volume is represented by the area of the smallest disk containing the model. The different models are obtained by setting different values for the carrying capacity parameter K in the logistic-growth function (equation 5), which controls the total growth of the cortical layer. If different models were simply scaled versions of one another, the perimeter of the cortical layer should vary as the square root of the area of the model. This is the case when the values of K produce non-convoluted models. However, when the models develop convolutions the growth of the cortical layer's perimeter dissociates from the growth of the total area, and the perimeter of the cortical layer is larger than expected by homothetic scaling. In Figure 4 the coefficient of the relation becomes closer to 1, and the perimeter increases almost linearly with the area. Figure 4 shows also that thinner cortical layers have more convolutions, and that these convolutions begin to develop earlier than for the thicker layers.
Cortical layer perimeter versus total area. Before convolutions develop, the perimeter grows proportionally to the square root of the area (slope 0.5 in the log–log graphic). This relation becomes close to linear when convolutions develop. Thinner cortices have more convolutions which begin to develop earlier (qh, thickness of the cortical layer; n, average number of convolutions). The graphic shows the perimeter versus area data points for 20 different growths (K between 0 and 160) and three different thicknesses of the cortical layer. Grey zones indicate data points belonging to a convoluted model.
Effect of Asymmetries on the Development and Distribution of Convolutions
Our model suggests that without any additional hypotheses the simple mechanical properties of the cortex are sufficient to produce cortical folding. Yet, the structural asymmetries induced by the early regionalization of the cerebral cortex can trigger the development of convolutions and influence their distribution. We have studied three types of asymmetries, illustrated in Figures 5–11:
Geometric asymmetries, which can be associated with the initial ‘bean-shape’ of the cortex before the period of rapid growth (Connolly, 1950). It has been observed that convolutions in fact appear to follow the global geometry of the brain, i.e. a longitudinal orientation in long brains and a transversal one in short brains (Le Gros Clark, 1945; Todd, 1982).
Mechanic asymmetries, which can be associated with the early differences in cortical cytoarchitecture. Regulatory genes have been found to express in discrete zones and gradients (Levitt et al., 1997; Rubenstein and Rakic, 1999) which could produce either an abrupt change or a more gradual variation in the type of tissue of two adjacent regions. In the adult brain many cytoarchitectonic areas with well-defined borders can be identified; however, the existence of gradual variations between cytoarchitectonic fields is controversial (Horton, 2000).
Growth asymmetries, which can be associated with the different growth dynamics of different cortical regions. For example, the growth of the striate cortex in the macaque is up to 2.5 times bigger than that of the adjacent extrastriate cortex (Rakic, 1988). Step differences in growth can also represent the decreased growth of a cortical region caused by the disruption of its normal afferents (Goldman-Rakic, 1980; Rakic, 1988; Dehay et al., 1996), or the regional disruption of cell proliferation observed in polymicrogyria (Piao et al., 2004; Rakic, 2004). Growth asymmetries can also be gradual, and antero-posterior and latero-medial gradients in the cortical proliferative kinetics have been identified at early stages in development (Sanderson and Weller, 1990; Levitt et al., 1997).
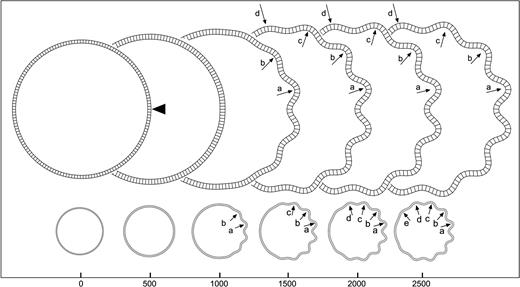
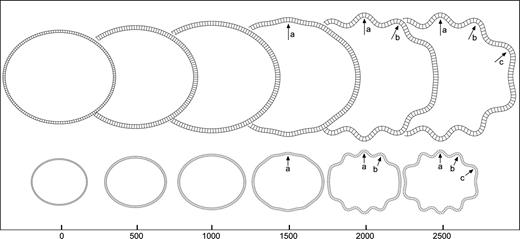
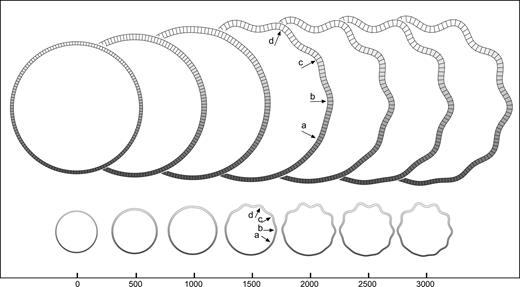
Geometric asymmetries: perturbation. Development of convolutions in the model where the point indicated by the arrowhead has been displaced to the centre by 0.01% of the total radius. The letters a, b, … signal the same convolution at different iteration steps. Elements = 150, qh = 4.5, qw = 4, kc = 0.5, τc = 4 × 104, kf = 0.05, τf = 104, K = 25, m = 5 × 10−3.
Geometric asymmetries: order of development. Evolution of convolutional development in the perturbed model of Figure 5. First convolutions to develop are deeper in the final configuration, and induce a cascade of smaller secondary and tertiary convolutions (in the order a, b, c, d, e).
Geometric asymmetries: elliptical shape. Development of convolutions in an elliptical model. Convolutions begin to develop at the minor axis of the model and propagate to the sides. The letters indicate the same convolutions throughout iteration steps. Elements = 150, qh = 4.5, qw = 4, kc = 0.5, τc = 4 × 104, kf = 0.05, τf = 104, K = 25, m = 5 × 10−3, major axis = 1.1 minor axis.
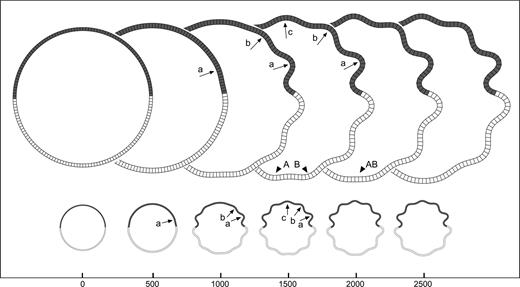
Mechanic asymmetries: step. Development of convolutions in the model with a step in the elastic constant of the cortical layer. The elastic constant of the cortical layer kc is smaller at the top of the model (more elastic). A convolution develops at the border and induces decreasing convolutions to the sides. A model with the same shape, growth, and mean elasticity does not develop detectable convolutions. Letters indicate the same convolution throughout iteration steps. Elements = 150, qh = 4.5, qw = 4, kc top = 0.4, kc bottom = 0.6, τc = 4 × 104, kf = 0.05, τf = 104, K = 20, m = 5 × 10−3.
Mechanic asymmetries: gradient. Development of convolutions in the model with a gradient in the elastic constant of the cortical layer (more elastic at top, i.e., smaller value of kc). Convolutions develop simultaneously and are more pronounced as the elasticity decreases. A model with the same shape, growth, and mean elasticity does not develop convolutions. Elements = 150, qh = 4.5, qw = 4, kc top = 0.4, kc bottom = 0.6, τc = 4 × 104, kf = 0.05, τf = 104, K = 20, m = 5 × 10−3.
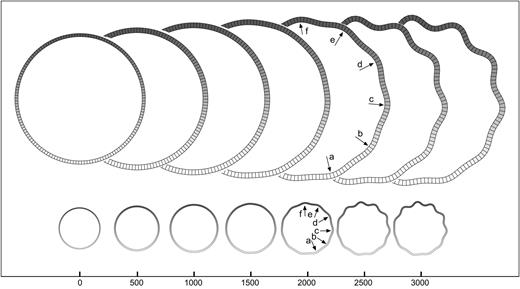
Growth asymmetries: step. Development of convolutions in the model with a step in the growth of the cortical layer (value of the parameter K). The growth is bigger at the top of the model. Convolutions develop at the side with more growth. A model with the same shape, mechanical properties, and mean growth does not develop convolutions. Elements = 150, qh = 4.5, qw = 4, kc = 0.5, τc = 4 × 104, kf = 0.05, τf = 104, K top = 20, K bottom = 5, m = 5 × 10−3.
Growth asymmetries: gradient. Development of convolutions in the model with a gradient in the growth of the cortical layer (smaller growth at bottom). Convolutions develop simultaneously and are less pronounced as the growth decreases. A model with the same shape, mechanical properties, and mean growth does not develop convolutions. Elements = 150, qh = 4.5, qw = 4, kc = 0.5, τc = 4 × 104, kf = 0.05, τf = 104, K top = 20, K bottom = 5, m = 5 × 10−3.
Geometric Asymmetries
Figure 5 illustrates the effect of the displacement of a single point of the cortical layer in the initial configuration of the model. The simulation shows that even a displacement which is 10−5 times the size of the radius of the model can induce the development of convolutions, and this with a set of mechanical and growth parameters that would otherwise produce a non-convoluted model. At the position of the perturbation (arrowhead in Fig. 5, iteration 0), a sulcus develops which triggers a cascade of convolutions towards the top and bottom halves. Figure 6 shows the evolution of the size of convolutions labelled a–d in Figure 5. At the end of the simulation the first convolutions to develop are the deepest ones, while those developing afterwards are progressively shallower. Convolutions are termed primary, secondary or tertiary, according to their time of development and final depth.
Figure 7 shows the effect of a global change in the initial geometry of the model. Instead of a circular initial shape, we have used an elliptical model. We observe the development of a cascade of convolutions beginning at the minor axis of the ellipse followed by convolutions of decreasing depth toward both sides. This kind of geometric asymmetry may induce the development of convolutions in an otherwise smooth model (convolutions are detected through the zero-crossings of the first derivative of the external contour of the cortical layer).
Mechanic Asymmetries
Figure 8 illustrates the effect of mechanic asymmetries on the model by introducing steps and gradients in the elastic constant of the cortical layer kc. For the mechanic step, the elastic constants of the top and bottom halves of the cortical layer have been set to different values (Fig. 8; the dark areas are more elastic, i.e. the value of kc is smaller). The simulation shows the development of a convolution at the border between both halves. This triggers a cascade of convolutions which become progressively smaller towards the sides being however deeper in the more elastic half. The exact location of the elasticity step is not in a sulcal fundus nor in a gyral crown, but at the inflexion.
The behaviour of the model with a top-down gradient in the elastic constant of the cortical layer kc is shown in Figure 9. We observe that convolutions develop simultaneously (approximately at iteration 2000) and that they are deeper in the less rigid half.
Growth Asymmetries
Figure 10 illustrates the effect of asymmetries in the growth of the cortical layer by introducing steps and gradients in the carrying capacity parameter K of the logistic-growth function. When a growth step is introduced between the top and bottom halves of the cortical layer, the first convolutions develop at the border between them, and then propagate to the sides developing progressively shallower convolutions. Convolutions are deeper in the region with more growth (Fig. 10, top) which contracts the region with less growth (bottom).
The effect of a top-down gradient in the carrying capacity of the cortical layer is shown in Figure 11. To the bottom, the zone of less growth develops less pronounced convolutions, which increase in depth as we move to the zone of more growth. The convolutions in the model develop simultaneously (approximately at iteration 1500).
Discussion
Cortical Growth and Development of Convolutions
The ontogeny of cortical gyrification in the mammalian brain follows three main stages: (i) a period of growth where the hemispheres remain smooth; (ii) a stage of rapid convolution development; and (iii) a long period of accommodation without changes in the degree of convolution (Connolly, 1950; Chi et al., 1977; Smart and McSherry, 1986a; Armstrong et al., 1995; Welker, 1990; Garel et al., 2001). These three stages are reproduced by our model simply by the action of cortical growth (Fig. 2A).
Our model suggests that cortical growth can induce the development of convolutions by itself, and does not require the specific folding mechanisms that have been proposed, such as resistance opposed by the cranium (Le Gros Clark, 1945), differential growth of the inner and outer cortical layers (Richman et al., 1975), cortical development genetically circumscribed to pro-gyral cortex (Welker, 1990) or specific corticocortical connections whose tension folds the cortex (Van Essen, 1997). Our results (see Fig. 3) support the view according to which the differences observed by Richman et al. (1975) in the surface area of the inner and outer cortical layers, which justifies their mechanical model, may be an effect, and not the cause of cortical folding (Van Essen, 1997). Furthermore, the apparent immobility of the sulcal fundi, reported by Smart and McSherry (1986a), and the differences in thickness between gyri and sulci, which represent a major justification for the gyrogenetic theory of Welker (1990), may also be an effect of growth-driven cortical folding. In Figure 2, the position of the sulcal fundi at the beginning of the simulation for the smooth model is almost the same as at the end, when convolutions have developed (see Fig. 2B2). Figure 3 shows that gyri can be significantly thicker than sulci for convolutions developed by cortical growth alone.
Many recent experimental studies have shown the importance of cortical growth for cortical folding and show, for example, that a global increase in cortical growth induces convolutions in the normally lissencephalic mouse brain (Haydar et al., 1999; Chenn and Walsh, 2002, 2003; Kingsbury et al., 2003). In our model, decreasing the growth of the cortical layer prevents the development of folds, while increasing the growth can induce folding in an otherwise smooth model.
Cortical Thickness and Degree of Convolution
The number and size of cortical convolutions vary in different gyrencephalic brains. The degree of convolution of a brain is frequently estimated according to the gyrification index introduced by Zilles et al. (1988). This index is defined as the ratio between the length of the pial contour and the length of the external contour in a coronal section. In our model, cortical growth plays a fundamental role in the development and deepening of convolutions, yet their number and size are determined by the thickness of the cortical layer. As illustrated in Figure 4, thin cortical layers develop more convolutions than thicker ones (which also begin to develop earlier). If the degree of convolution of our model were to be studied according to the gyrification index, this parameter would not discern between the effect of cortical growth and thickness. In fact, the gyrification index does not allow us to distinguish between a cortex with a small number of deep convolutions and one with a large number of superficial convolutions. Based on our model, we suggest that a measure of the relative thickness, such as the ratio between the mean cortical thickness and the external contour length, should effectively complement the gyrification index.
Evolutionary Variations in the Degree of Convolution
The allometric study of different parameters such as body size, brain size and white matter volume among different mammalian species is a major tool for investigating brain evolution (Jerison, 1973). In particular, allometric studies show that in gyrencephalic brains the cortical surface area increases almost proportionally to the brain volume. This relationship reveals a trend towards the expansion of the cerebral cortex in larger mammals (Rakic, 1988; Caviness et al., 1995; Kornack and Rakic, 1998). The increased degree of convolution of larger brains results in an increased cortical surface without a comparable increase in the total cerebral volume. Our model is able to reproduce this relationship, and the increase in the cortical layer's perimeter of a convoluted model is larger than for a smooth model of equal total area (see Cortical Surface and Brain Volume).
There are cases, however, where the linear relationship between cortical surface area and brain volume does not applies. A well-known exception is the manatee, a large marine mammal with an almost lissencephalic cortex. The lack of convolutions in the manatee brain can be explained by the dependence of the degree of convolution on cortical thickness, as suggested by our model. The manatee's cortex is in fact exceptionally thick, with a mean cortical thickness of 4 mm (Reep and O'Shea, 1990) — compared, for example, with the human brain, whose mean cortical thickness is 2.5 mm (Fischl and Dale, 2000). On the other hand, cetaceans such as whales and dolphins have thin and profusely convoluted cortices, in agreement with our results. Experimental support for the idea of the dependence of the degree of convolution on cortical thickness can be found in the results of allometric studies of Hofman (1991) and Prothero (1997). The relationship between cortical surface area and brain volume presents a strong correlation. Cortical volume (associated with cortical growth) is the parameter which most strongly correlates with brain volume, while the weakest correlation is observed for the mean cortical thickness. This may indicate that the main linear trend in the evolution of surface area depends on cortical growth, while the most plausible source of variability is the mean cortical thickness.
Early Regionalization, Cortical Geometry and Organization of Convolutions
The steady growth of the cortical layer in the morphogenetic model produces convolutions of the same size and with almost the same time of development. This type of folding is different from that observed in gyrencephalic brains, which show gradients in the degree of convolution and a hierarchical organization of sulci with differences in their sizes and onset time. Our results suggest that this organization can be produced by early asymmetries (gradual or abrupt changes) in the geometry, mechanical properties and growth dynamic of the cerebral cortex, which can induce and guide the development of convolutions. To our knowledge, the morphogenetic model is the only model of gyrification that reproduces these phenomena.
Gradients in the degree of convolution have been reported in the brains of many species of primates (Zilles et al., 1989), dogs (Wosinski et al., 1996) and also humans (Zilles et al., 1988; Armstrong et al., 1995), yet the development of convolutions seems to begin at almost the same time for all the cortical regions (Chi et al., 1977; Armstrong et al., 1995; Garel et al., 2001). This simultaneous development of convolutions with graded sizes is reproduced by our model as the effect of a gradient in the mechanical properties or in the growth of the cortical layer (see Figs 9 and 11). Many studies have reported developmental gradients that appear to play an important role in the definition of cortical architecture (Berry and Rogers, 1965; Rakic, 1976; Smart, 1983; Sanderson and Weller, 1990; Simeone et al., 1992; Donoghue and Rakic, 1999; Nakagawa et al., 1999; Fukuchi-Shimogori and Grove, 2001). Our model suggests a link between these early developmental gradients and the gradients in the degree of convolution observed in the adult cortex.
Sulci can be hierarchically classified into primary, secondary and tertiary depending of the differences in size and time of development (Chi et al., 1977; Ono et al., 1990; Armstrong et al., 1995; Garel et al., 2001). This hierarchical organization of sulci is reproduced by our model as the effect of abrupt changes in the mechanical properties or in the growth of the cortical layer, and also as the effect of early asymmetries in its geometry (see Figs 5–8 and 10), i.e. our model suggests that the position of primary convolutions may be determined both by the location of architectonic inhomogeneities or by the initial geometry of the cortex. The secondary and tertiary convolutions that subsequently develop are an effect of the preceding primary convolutions. Their position is not necessarily related to architecture.
The correlation between the functional and architectonic organization of the cortex and the gyral/sulcal anatomy has been a subject of long debate. On the one hand, several studies have proposed a close relationship, based, for example, on the structural differences between gyral and sulcal cortex (Welker, 1990) or on the strong correlation between sulcal anatomy and functional domains in the somatosensory cortex of rodents' forepaw (Woolsey, 1960; Welker and Campos, 1963). On the other hand, based on results obtained by the quantitative characterization of cortical cytoarchitecture, it has been proposed that the relationship is enormously variable (Zilles et al., 1997). Our model suggests that a relationship between cortical architecture and folding pattern exists, but that it is more complex than a simple match between sulcal fundi and architectonic boundaries. The relationship is also different for primary convolutions than it is for the remaining secondary and tertiary convolutions. Furthermore, our model suggests that primary convolutions can also develop as the effect of geometrical asymmetries, independently of architectonic variations of the cortical tissue.
The possibility of the independence between the development of a primary convolution and cytoarchitecture raises further questions on the processes underlying the relationship between anatomy, architecture and connectivity. Current results concerning the genetic control of cortical regionalization suggest that cytoarchitectonic areas are defined in part by the early diffusion of regulatory genes and molecular signals (Fukuchi-Shimogori and Grove, 2001, 2003), which can also participate in the development of thalamocortical connections (O'Leary and Nakagawa, 2002; López-Bendito and Molnár, 2003). Convolutions developing at these early stages may introduce important large-scale geometric anisotropies for the diffusion of these signals. Cortical folding and regionalization are concomitant processes, and while our model only studies the effect of cortical architecture on cortical folding, it is theoretically possible that cortical folding may have an effect on the definition of cytoarchitectonic regions.
Finally, the possibility of the development of primary sulci independently from cytoarchitecture suggests a complement to the classical concept of sulcal homology. The establishment of sulcal homologies is important for the comparison of brains among species, and then for the study of the evolution of the cortical organization. When sulci are compared, they can be said to be homologous if they limit the same cortical area. Connolly (1950) has already highlighted the difficulties of this definition. The lunate sulcus, for example — a major landmark of the primate occipital lobe — appears to limit precisely the striate cortex in cercopithecus, yet the same sulcus in the orangutan or in the chimpanzee does not show such a close relationship to cytoarchitecture. Furthermore, the lunate sulcus develops in its normal position in enucleated macaques, where the striate/extrastriate limit is far displaced to the occipital pole (Dehay et al., 1996). If, as we suggest, primary convolutions can be induced by the initial shape of the cortical surface and therefore independently of cytoarchitecture, we must distinguish between two types of sulcal homology: those sulci related to the same architectonic invariant and those related to the same geometrical invariant in the cortical anatomy. The simulation of the development of convolutions in a three-dimensional implementation of the morphogenetic model where the initial geometry resembles the early bean shape of the developing brain should help to analyse this question.
Afferent Connectivity and Convolutional Development
Two theories have been proposed which can explain the role of thalamocortical and corticocortical afferents on the development of convolutions. The first suggests that the mechanical tension produced by these fibres is the primary driving force for convolution development (Welker, 1990; Dehay et al., 1996; Van Essen, 1997). In the macaque brain the border between striate and extrastriate cortex lies on the gyrus where the operculum and the posterior bank of the lunate sulcus limit. According to the tension-based theory, the development of this gyrus is explained by the massive reciprocal connections between these two regions, as tension pulls these strongly interconnected regions towards one another (Van Essen, 1997). However, as we remarked before, the position of this gyrus and of the lunate sulcus remains the same in enucleates where the cytoarchitectonic border is far shifted, which suggests that a different mechanism produces it. Indeed, Goldman and Galkin (1978) reported that primary sulci, such as the lunate sulcus, can be observed in the macaque brain as dimples by embryonic days (E)105–107 and are already evident as sulci by E119, which is before corticocortical afferents begin to innervate the cortex, roughly between E124 and E150 (Goldman-Rakic, 1980). Furthermore, in purely mechanical terms, the disruption of thalamocortical fibres or corticocortical fibres should produce less tension, and consequently less deep or even absent sulci, but in fact experimental evidence shows an overproduction (Goldman-Rakic, 1980; Rakic, 1988; Dehay et al., 1996).
An alternative theory was first proposed by Goldman-Rakic (1980) based on the important effect of afferent fibres on the maturation of the cerebral cortex (Goldman-Rakic, 1980; Dehay et al., 1996, 2001; Edgar and Prince, 2001). The absence of normal thalamocortical afferents, for example, is associated with the atrophy of cortical cells, the increase of normal cell death, the reduction of cell generation and the disruption of the normal migration of cells to the cortical plate (Goldman-Rakic, 1980; Dehay et al., 1996, 2001). The disruption of thalamocortical fibres in the early enucleate (Rakic, 1988, 1991; Dehay et al., 1989, 1996) and corticocortical fibres in the early resection of the dorsal prefrontal cortex in macaques (Goldman and Galkin, 1978; Goldman-Rakic, 1980) depress the growth of their target regions which may then alter its normal gyrification (overproduction of ectopic sulci). The morphogenetic model suggests a mechanism by which the modulatory influence of afferent fibres on cortical growth can produce a modification of gyrification. In Figure 10, the differences in growth between the top and bottom halves of the cortical layer induce the formation of convolutions in a model that should be smooth if growth was homogeneous.
Conclusion
There is a rich literature of detailed descriptions of the ontogeny and anatomy of cortical convolutions. However, very few formal models have been proposed to explain their development, and the mechanisms involved in the gyrification of the cortex remain poorly understood. In this article we have presented a simple and parsimonious model to study the main factors involved in the folding of the cerebral cortex. Despite its simplicity, the morphogenetic model is able to give a unified interpretation for many phenomena concerning convolutional development, in particular, the role of mechanic and genetic factors. Yet, as the adult pattern of convolutions seems to be determined by the action of many different processes (Welker, 1990), many important characteristics of convolutional development do not lie within the scope of our simple, two-dimensional implementation of the morphogenetic model. A three-dimensional implementation, and a more detailed account of connectivity, should allow us to address problems such as the organization of convolutions over the cortical surface, the effect of cortical folding on the diffusion of molecular signals, and the relationship between corticocortical connectivity and the sulcal/gyral anatomy (Van Essen, 1997). Another important improvement of the implementation would be to manage the mechanical contact. At present, nothing prevents the self-intersection of the cortical layer or of the radial elements, which limits the maximum growth that we can study. Early in development, gyri are rounded with open sulci, then the pressure exerted by the skull makes sulcal walls become closely opposed and gyral crowns flattened (Smart and McSherry, 1986a). The detection of mechanical contact should allow us to simulate better the materiality of gyri and thus to approximate the shape of real cortical convolutions better.
The analysis and the comparison of folding patterns is of landmark relevance for different fields in neuroscience: it provides important insights into the evolution of the mammalian cortex, it is fundamental for the study and diagnosis of multiple developmental malformations and degenerative syndromes, and it is a central problem in the analysis of neuroimaging data. The morphogenetic model provides a theoretical framework for the interpretation of many aspects of cortical folding. Finally, a relationship between convolutional anatomy and the organization of cortical architecture and function seems beyond doubt, and the morphogenetic model represents a first step towards a theoretical analysis of this question.
We thank E. Carey, C. Lienhart, F. Aboitiz, J-P Aubin and H. Kennedy for their help in reading the original manuscripts. RT has been supported by Scito S.A.
References
Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings H, Zilles K (
Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K (
Barron D (
Berry M, Rogers A (
Biondi A, Nogueira H, Dormont D, Duyme M, Hasboun D, Zouaoui A, Chantome M, Marsault C (
Bishop K, Goudreau G, O'Leary D (
Caviness V Jr, Takahashi T, Nowakowski R (
Chada S, Lamoureux P, Buxbaum R, Heidemann S (
Chenn A, Walsh C (
Chenn A, Walsh C (
Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H (
Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H (
Dehay C, Giroud P, Berland M, Killackey H, Kennedy H (
Dehay C, Savatier P, Cortay V, Kennedy H (
Donoghue M, Rakic P (
Edgar J, Price D (
Elliot Smith G (
Fischl B, Dale A (
Fukuchi-Shimogori T, Grove E (
Fukuchi-Shimogori T, Grove E (
Garel C, Chantrel E, Brisse H, Elmaleh M, Luton D, Oury J, Sebag G, Hassan M (
Garel S, Huffman K, Rubenstein J (
Geyer S, Schleicher A, Zilles K (
Goldman P, Galkin T (
Goldman-Rakic P (
Hasnain M, Fox P, Woldorff M (
Haydar T, Kuan C, Flavell R, Rakic P (
Jerison H (
Job C, Tan S (
Kingsbury M, Rehen S, Contos J, Higgins C, Chun J (
Kornack D, Rakic P (
Krubitzer L, Kahn D (
Le Gros Clark W (
Levitt P, Barbe M, Eagleson K (
López-Bendito G, Molnár Z (
Malamud N, Hirano A (
Miyashita-Lin E, Hevner R, Wassarman K, Martinez S, Rubenstein J (
Nakagawa Y, Johnson J, O'Leary D (
O'Leary D, Nakagawa Y (
Ono M, Kubik S, Abernathy C (
Pallas S (
Piao X, Hill R, Bodell A, Chang B, Basel-Vanagaite L, Straussberg R, Dobyns W, Qasrawi B, Winter R, Innes A, Voit T, Ross M, Michaud J, Déscarie J, Barkovich A, Walsh C (
Prothero J, Sundsten J (
Rademacher J, Morosan P, Schormann T, Schleicher A, Werner C, Freund H, Zilles K (
Rakic P (
Rakic P (
Rakic P (
Reep R, O'Shea T (
Richman D, Stewart R, Hutchinson J, Caviness V Jr (
Roland P, Zilles K (
Sanderson K, Weller W (
Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E (
Smart I, McSherry G (
Smart I, McSherry G (
Todd P (
Van Essen D (
Welker W (
Welker W, Campos G (
White T, Andreasen N, Nopoulos P (
Woolsey C (
Wosinski M, Schleicher A, Zilles K (
Zhu C, Bao G, Wang N (
Zilles K, Armstrong E, Schleicher A, Kretschmann H (
Zilles K, Armstrong E, Moser K, Schleicher A, Stephen H (
Zilles K, Schleicher A, Langemann C, Amunts K, Morosan P, Palomero-Gallagher N, Schormann T, Mohlberg H, Burgel U, Steinmetz H, Schlaug G, Roland P (


![Finite-elements model of the developing cerebral hemisphere. (A) Initial shape of the finite-elements model. The cortical layer is composed of continuous quadrilateral elements attached to the centre by linear radial elements. (B) Dimensions of the finite-elements model. The initial length of the radial elements is rf0, the initial size of the quadrilateral elements of the cortical layer is qh in the radial direction and qw in the tangential direction. (C) Calculation of the deformation forces in the quadrilateral elements. The forces in each quadrilateral element are calculated as the difference between the actual deformed configuration and the rest configuration of the quadrilateral translated in tdr and rotated in θdr. Coordinates of the rest configuration: \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{0}^{1},\) \end{document}\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{0}^{2},\) \end{document}\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{0}^{3}\) \end{document} and \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{0}^{4}.\) \end{document} Coordinates of the deformed configuration: c1, c2, c3 and c4. Coordinates of the translated and rotated rest configuration: \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{tr}^{1},\) \end{document}\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{tr}^{2},\) \end{document}\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{tr}^{3}\) \end{document} and \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(c_{tr}^{4}.\) \end{document}](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cercor/15/12/10.1093/cercor/bhi068/2/m_cercorbhi068f01_ht.jpeg?Expires=1716445825&Signature=nCmwvjS64oGh6OHgCUFZlNYdJVwWDayg-WQKeWzARvB3Hu25I2pcOdp5evMWI1vG~6RMQqMcVu~o0P2uW5NxOb9lKpQTEv4rfNIVu8PDC0o98HHBecJZqggYPYujd5pHZsuGX6qBVXn7ekFZEkPobRLKyk8XgqLJ319ANegvXDc~5IQlQxra1UEXuZl4pyDHfDEEHPBT1nTIkQ4AB-87-hIcYIQpHw4yQiWVZ9Mp1I~4~BSK5TBUFfuWFlWiUDUdo2OeNEoMtCX1k9c8yOz-O0xcvsUCLhNyj5JOaQYeSammUiOJvQ1DOMmpa5rYvEhZtiofELMlnACYqG6GJdbaCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)