-
PDF
- Split View
-
Views
-
Cite
Cite
Tiziana Antonelli, Maria Cristina Tomasini, Maria Tattoli, Tommaso Cassano, Sergio Tanganelli, Simone Finetti, Elisa Mazzoni, Luigia Trabace, Luca Steardo, Vincenzo Cuomo, Luca Ferraro, Prenatal Exposure to the CB1 Receptor Agonist WIN 55,212-2 Causes Learning Disruption Associated with Impaired Cortical NMDA Receptor Function and Emotional Reactivity Changes in Rat Offspring, Cerebral Cortex, Volume 15, Issue 12, December 2005, Pages 2013–2020, https://doi.org/10.1093/cercor/bhi076
Close - Share Icon Share
Abstract
The aim of this study was to investigate whether prenatal exposure to the cannabinoid CB1 receptor agonist WIN 55,212-2 (WIN) at a daily dose devoid of overt signs of toxicity and/or gross malformations (0.5 mg/kg, gestation days 5–20), influences cortical glutamatergic neurotransmission, learning and emotional reactivity in rat offspring. Basal and K+-evoked extracellular glutamate levels were significantly lower in cortical cell cultures obtained from pups exposed to WIN during gestation with respect to those measured in cultures obtained from neonates born from vehicle-treated dams. The addition of NMDA to cortical cell cultures from neonates born from vehicle-treated dams concentration-dependently increased glutamate levels, and this was absent in cell cultures obtained from WIN-exposed pups. WIN-exposed rats also revealed a poorer performance in homing (10–12 days of age) and active avoidance tests (80 days of age) as well as a decrease in the rate of separation-induced ultrasonic emission (10 days of age). Finally, prenatal exposure to WIN induced a reduction in the number of cortical neuronal population. These findings (i) provide evidence for a deficit in cortical glutamatergic neurotransmission and behaviour in the rat neonate following prenatal exposure to WIN; and (ii) suggest that the reduction in cortical glutamatergic neurotransmission, NMDA receptor activity and alterations in neuronal development might underlie, at least in part, the learning deficit and decreased emotional reactivity observed in the offspring.
Introduction
The recreational use of marijuana in western society is not only found among young adults but is also increasingly used by pregnant women (Day et al., 1994; Rubio et al., 1998; Fried and Smith, 2001). This increase in consumption may reflect the popular conception that the use of marijuana is virtually free of noxious side effects. Moreover, the scientific literature on the effects of prenatal marijuana exposure is controversial and far from definitive (Fried and Smith, 2001). Cannabinoid CB1 receptors are present during prenatal development and cannabinoids can be transferred from the mother to the offspring through the placental blood during the gestation in rodents and human (Dalterio, 1986). Recent studies reported that the main psychoactive component of marijuana, delta9-tetrahydrocannabinol (Δ9-THC), interferes with the rodent ontogenic processes, particularly the dopaminergic (Hernandez et al., 1997) and enkephalinergic neuron (Vela et al., 1998) development. Furthermore, prenatal marijuana exposure alters cognitive function in mammals (Navarro et al., 1995; Paule et al., 1998), a hypothesis supported by the findings that learning/memory impairments are the most commonly reported behavioural effects associated with marijuana consumption (Ameri, 1999; Hampson and Deadwyler, 1999). In particular, the use of marijuana alters hippocampal function, thereby inducing memory deficit (Lichtman and Martin, 1996). The cellular/molecular mechanisms that underlie these behavioural effects may lead to an inhibition of long-term potentiation (LTP) in the hippocampus CA3–CA1 region (Terranova et al., 1995; Stella et al., 1997; Misner and Sullivan, 1999) and to a depression of local glutamatergic neurotransmission (Shen et al., 1996; Sullivan, 1999). In fact, we have demonstrated (Mereu et al., 2003) that prenatal exposure to the CB1 receptor agonist WIN 55,212-2 (WIN) induced a disruption of memory retention in offspring subjected to a passive avoidance task. This memory impairment was correlated with alterations in hippocampal LTP and glutamate release.
To further investigate the mechanism(s) underlying the neurobiological substrate of cognitive alterations deriving from prenatal cannabinoid exposure, and in view of (i) the role of the cerebral cortex in cognitive processes (Aggleton et al., 1995; Ragozzino et al., 1998), (ii) the presence of CB1 receptor in the cerebral cortex of the foetus and (iii) the role of glutamate in cortical function (Auclair et al., 2000), the effects of prenatal WIN administration on cortical glutamate levels, NMDA receptor function and neuronal population were investigated by employing cortical cell cultures from rat pups born from a mother who had received a daily 0.5 mg/kg dose of WIN during pregnancy. Moreover, to evaluate the influence of prenatal WIN exposure on the offspring's learning ability during the early phase of postnatal life, homing behaviour, a simple form of learning based on ultrasonic and olfactory cues (Annau and Cuomo, 1988; Bignami, 1996), was analysed in 6- to 12-day-old pups. Learning function (as evaluated in an active avoidance task) was also explored in 80-day-old rats. Finally, since glutamatergic neurotransmission is involved in rat emotional reactivity (Kehne et al., 1991), we investigated the effects of prenatal WIN exposure on separation-induced ultrasonic emission, a valuable indicator of emotionality in rat pups (Insel et al., 1986).
Materials and Methods
Animal Care
All experiments were performed in accordance with the guidelines issued by the Italian Ministry of Health (D.L. 116/92) and (D.L. 111/94-B), the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institute of Health (USA).
Animals and Exposure Conditions
For 1 week prior to mating, primiparous Wistar female rats (Harlan SRC, Milan, Italy) weighing 250–280 g were housed at constant room temperature (20 ± 1°C) and humidity (60%), and exposed to a light cycle of 12 h/day (08:00 to 20:00 h), with food and water available ad libitum. For mating, pairs of females were placed with single male rats in the late afternoon. Vaginal smears were taken the following morning at 09:00 h. The day on which sperm were present was designated as the day 0 of gestation (GD 0).
Treatment was performed daily with WIN (0.5 mg/kg) or WIN vehicle, from GD 5 to GD 20. The drug was suspended in 0.3% Tween 80–saline as described previously (Mereu et al., 2003), and injected s.c. at a volume of 1 ml/kg. Control rats were injected with the vehicle.
This dose was chosen on the basis of our pilot studies, which showed that prolonged prenatal exposure to a higher WIN dose (1.0 mg/kg) significantly affected reproduction parameters such as dam and pup weight gain, as well as litter size at birth (Mereu et al., 2003).
Litters were reduced to a standard size of six male pups (when possible) within 24 h after birth. Litters from vehicle and WIN-exposed dams were then assigned to non-exposed mothers whose pups were born on the same day. One pup per litter from different treatment groups was then used in each experiment. Pups were weaned at 21 days of age. Each male pup was used for a single test and tested only once.
Reproduction Data
Body weights of each dam was taken on GD 0 and 20. The number of dams giving birth and the length of pregnancy were determined. Litter size at birth, pup weight gain and postnatal mortality (number of male pups that died before weaning) were evaluated.
Neurochemical Experiments
Cortical Cell Cultures Preparation
A cerebral cortical cell culture was prepared from 1 day-old neonates (Alho et al., 1988). After the resuspension in the plating medium, the cells were counted and then plated on poly-L-lysine (5 μg/ml)-coated dishes at a density of 2.5 × 106 cells/dish. The plating medium consisted of Eagle's basal medium supplemented with inactivated foetal calf serum, 25 mM KCl, 2 mM glutamine and 100 μg/ml gentamycine. Cultures were grown at 37°C in a humidified atmosphere, 5% CO2/95% air. Cytosine arabinoside (10 μM) was added within 24 h of plating to prevent glial cell replication. The cultures were used in experiments after an 8 day incubation in vitro.
Experimental Protocol
On the day of the release experiment, the cells were rinsed twice by replacing the culture medium with a warmed (37°C) Krebs' Ringer–bicarbonate buffer (in mM: NaCl 118.5, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, NaH2PO4 1.2, glucose 11, pH 7.4). Thereafter, five consecutive fractions were collected renewing this solution (400 μl) every 30 min. The first two samples were used to assess basal glutamate levels while, to evoke endogenous glutamate, the cells were treated with an isotonic Krebs solution containing 20 mM KCl, applied 20 min before the end of the third fraction. A pharmacological challenge with the glutamate receptor agonist N-methyl-D-aspartic acid (NMDA, 0.01–10 μM, 10 min) was carried out during the third fraction by applying the compound 10 min before the end of the collection period. The content of protein per dish was determined according to Bradford (1976).
Endogenous Glutamate Assay
Endogenous glutamate was quantified by using a high performance liquid chromatography/fluorimetric detection system, including precolumn derivatization o-phthaldialdehyde reagent and a Chromsep 5 (C18) column. The mobile phase consisted of 0.1 M sodium acetate, 10% methanol and 2.5% tetrahydrofurane, pH 6.5. The limit of detection for glutamate was 30 fmol/sample.
Behavioural Experiments
Homing Behaviour
The Homing test used in the present study was a modified version of that recently described by Cagiano et al. (2002). Briefly, the apparatus consisted of a transparent polycarbonate cage (26 × 42 × 30 cm) divided in three equal in size compartments: a central ‘test arena’ and two lateral compartments, with the opposite sides surrounded by a wire fence and positioned between an experimental home cage (containing mother and siblings with nest bedding) or an empty cage (containing fresh bedding).
Each compartment was divided into four identical sectors. The outer sectors of the home side compartment was defined as the ‘goal area’ (26 × 7 cm). Each pup was removed from its home cage and placed in the centre of the ‘test arena,’ randomly right or left at the ‘goal area’. Homing behaviour was videotaped using a video camera and a monitor. The latency (in s) to place both forelimbs in the goal area was recorded in a 180 s session.
One pup per litter from each treatment group (vehicle: n=12; WIN: n=10) was tested on postnatal days (PNDs) 6, 8, 10 and 12.
Ultrasonic Vocalization
Recording sessions were conducted in a sound-attenuating cabin (Amplifon G-type, 1.00 × 1.00 × 2.00 m) according to the technique previously described by Cagiano et al. (1988). Vocalizations of rat pups were recorded on a Racal Store 4DS high-speed tape recorder using a direct mode recording procedure with a tape speed of 30 in/s (76.8 cm/s); the frequency response range was flat between 200 Hz and 150 kHz. Ampex magnetic tapes (length = 3.280 ft/100 cm, width = 0.25 in/0.64 cm) with precision reels were used. The transducers employed were a calibrated Bruel & Kjaer (model 4135) 0.25 in (0.64 cm) free-field condenser microphone (frequency response flat within ±3 dB, from 4 to 100 kHz), a Bruel & Kjaer microphone preamplifier (model 2618) with a linear frequency response from 10 to 200 kHz, which provided 20 dB amplification, a Rank Precision Ind. low-noise amplifier, which provided 20 dB amplification steps, and a Krohn-Hite tunable band-pass filter (model 3500) set at 20–100 kHz.
The rate of vocalization (number of calls/15 s) was counted manually by listening to the audible output of the tape recorder with a loudspeaker and by watching the vocalization displayed on Bruel & Kjaer (model 2033) high-resolution signal analyser on time-intensity mode during tape replay at 3.75 in/s.
One of the eight male pups in each litter was randomly removed from the nest (one pup per litter from eight different litters) and placed in a shallow glass dish (15 cm in diameter and 10 cm deep) 15 s before the test. This confined the pup relative to the microphone, which was supported vertically 15 cm above the dish and thus avoided handling it during the recording session, which lasted 15 s. Ultrasonic vocalization (15 s/session) was evaluated at PND 10. Each group consisted of eight male pups.
Active Avoidance Behaviour
The apparatus and procedures have been previously described (De Salvia et al., 1995). Briefly, the apparatus consisted of a two-way avoidance box housed inside a sound-attenuating chamber (Amplifon G-type cabin). Each avoidance box was divided into two compartments connected by an opening of 9 × 12 cm and operated by electromechanical programming equipment. The conditioned stimulus (CS) consisted of a light (3 W lamp) that was alternately switched on in each compartment. Onset of the CS was followed 12 s later by the unconditioned stimulus (US), which was a 0.5 mA scrambled foot shock. The CS remained on during the presentation of the US, which lasted a maximum of 18 s, when both the CS and US were turned off.
A conditioned avoidance response (CAR) was recorded when an animal avoided the US by crossing over to the opposite compartment during the first 12 s when only the CS was on. An escape response consisted of the animal moving into the opposite compartment following the onset of the US. This response terminated both the CS and the US.
Eighty-day-old males were subjected daily (for 20 consecutive days) to a 20 trial session, with a 60 s intertrial interval, for 20 days. Rats reaching the criterion of 80% of CARs for three consecutive sessions were considered conditioned. Each group consisted of 10 animals.
Morphological Experiments
Immunocytochemistry
On DIV 8, cells were rinsed in 0.1 M phosphate-buffered saline (PBS) and then fixed using 4% paraformaldehyde in Sorensen's buffer 0.1 M, pH 7.4, for 20 min. After rinses in PBS (three times for 5 min each), the cells were incubated overnight at 4°C with the primary antibody rabbit anti-microtubule associated protein-2 (MAP2). Anti-MAP2 antibody was diluted 1:1000 in PBS containing 0.3% Triton X-100 (v/v). The cells were then washed three times with PBS and incubated with rhodamine-conjugated antirabbit antibody (Chemicon) diluted 1:100 in PBS containing 0.3% Triton X-100 for 60 min at room temperature. After three washes in PBS, the cells were mounted in glycerol and PBS (3:1, v/v) containing 0.1% 1,4-phenylenediamine, and examined using a Nikon Microphot FXA microscope.
Statistical Analysis
During all the experiments, the researchers have been blind to the condition (i.e. prenatal treatment) of the tested animals. The reproduction data were analysed by overall one-way or two-way analyses of variance (ANOVAs) followed by post-hoc tests (Tukey's test) for individual comparisons between groups; Fisher's exact-test was used where appropriate. The effects of the NMDA and KCl treatments on endogenous extracellular glutamate levels during the third fraction were reported as percent change of basal values calculated as the mean of the two fractions collected prior treatment. Data obtained from basal and KCl application were analysed by Student's t-test for grouped data. The statistical analysis for NMDA treatments was carried out by one-way ANOVA followed by the Newman–Keuls test for multiple comparisons. The statistical analysis have been performed on the basis of cell dish numbers (Table 1).
Numbers of analysed cell cultures, animals and pregnancies in each set of in vitro experiment
| Figure . | Experiment . | Group . | No. of dishes . | No. of pups . | No. of pregnancies . |
|---|---|---|---|---|---|
| 1 | Basal glutamate levels | Vehicle | 28 | 10 | 5 |
| WIN | 31 | 10 | 5 | ||
| 2 | Evoked glutamate levels | Vehicle | 32 | 10 | 55 |
| WIN | 35 | 10 | |||
| 3 | Control | Vehicle | 25 | 10 | 5 |
| WIN | 26 | 10 | 5 | ||
| 3 | 0.01 nM NMDA | Vehicle | 15 | 8 | 4 |
| WIN | 15 | 8 | 4 | ||
| 3 | 0.1 nM NMDA | Vehicle | 28 | 8 | 4 |
| WIN | 24 | 8 | 4 | ||
| 3 | 1 nM NMDA | Vehicle | 32 | 8 | 5 |
| WIN | 33 | 8 | 5 | ||
| 3 | 10 nM NMDA | Vehicle | 31 | 10 | 5 |
| WIN | 30 | 9 | 5 | ||
| 5 | MAP 2 | Vehicle | 12 | 5 | 5 |
| WIN | 12 | 5 | 5 |
| Figure . | Experiment . | Group . | No. of dishes . | No. of pups . | No. of pregnancies . |
|---|---|---|---|---|---|
| 1 | Basal glutamate levels | Vehicle | 28 | 10 | 5 |
| WIN | 31 | 10 | 5 | ||
| 2 | Evoked glutamate levels | Vehicle | 32 | 10 | 55 |
| WIN | 35 | 10 | |||
| 3 | Control | Vehicle | 25 | 10 | 5 |
| WIN | 26 | 10 | 5 | ||
| 3 | 0.01 nM NMDA | Vehicle | 15 | 8 | 4 |
| WIN | 15 | 8 | 4 | ||
| 3 | 0.1 nM NMDA | Vehicle | 28 | 8 | 4 |
| WIN | 24 | 8 | 4 | ||
| 3 | 1 nM NMDA | Vehicle | 32 | 8 | 5 |
| WIN | 33 | 8 | 5 | ||
| 3 | 10 nM NMDA | Vehicle | 31 | 10 | 5 |
| WIN | 30 | 9 | 5 | ||
| 5 | MAP 2 | Vehicle | 12 | 5 | 5 |
| WIN | 12 | 5 | 5 |
Numbers of analysed cell cultures, animals and pregnancies in each set of in vitro experiment
| Figure . | Experiment . | Group . | No. of dishes . | No. of pups . | No. of pregnancies . |
|---|---|---|---|---|---|
| 1 | Basal glutamate levels | Vehicle | 28 | 10 | 5 |
| WIN | 31 | 10 | 5 | ||
| 2 | Evoked glutamate levels | Vehicle | 32 | 10 | 55 |
| WIN | 35 | 10 | |||
| 3 | Control | Vehicle | 25 | 10 | 5 |
| WIN | 26 | 10 | 5 | ||
| 3 | 0.01 nM NMDA | Vehicle | 15 | 8 | 4 |
| WIN | 15 | 8 | 4 | ||
| 3 | 0.1 nM NMDA | Vehicle | 28 | 8 | 4 |
| WIN | 24 | 8 | 4 | ||
| 3 | 1 nM NMDA | Vehicle | 32 | 8 | 5 |
| WIN | 33 | 8 | 5 | ||
| 3 | 10 nM NMDA | Vehicle | 31 | 10 | 5 |
| WIN | 30 | 9 | 5 | ||
| 5 | MAP 2 | Vehicle | 12 | 5 | 5 |
| WIN | 12 | 5 | 5 |
| Figure . | Experiment . | Group . | No. of dishes . | No. of pups . | No. of pregnancies . |
|---|---|---|---|---|---|
| 1 | Basal glutamate levels | Vehicle | 28 | 10 | 5 |
| WIN | 31 | 10 | 5 | ||
| 2 | Evoked glutamate levels | Vehicle | 32 | 10 | 55 |
| WIN | 35 | 10 | |||
| 3 | Control | Vehicle | 25 | 10 | 5 |
| WIN | 26 | 10 | 5 | ||
| 3 | 0.01 nM NMDA | Vehicle | 15 | 8 | 4 |
| WIN | 15 | 8 | 4 | ||
| 3 | 0.1 nM NMDA | Vehicle | 28 | 8 | 4 |
| WIN | 24 | 8 | 4 | ||
| 3 | 1 nM NMDA | Vehicle | 32 | 8 | 5 |
| WIN | 33 | 8 | 5 | ||
| 3 | 10 nM NMDA | Vehicle | 31 | 10 | 5 |
| WIN | 30 | 9 | 5 | ||
| 5 | MAP 2 | Vehicle | 12 | 5 | 5 |
| WIN | 12 | 5 | 5 |
Homing behaviour data was analysed by a two-way ANOVA followed by Tukey's test for individual comparisons. Ultrasonic vocalizations were analysed by Student's t-test. Active avoidance behaviour data was evaluated by Fisher's exact test.
Substances
WIN 55,212-2 mesylate ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinyl-methyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone) (Tocris Cookson Ltd, Bristol, UK) was suspended in 0.3% Tween 80-saline. NMDA, was purchased from Sigma Chemical Co. (St Louis, MO). The culture dishes were purchased from NUNC A/S, Denmark. Foetal calf serum and basal Eagle's medium were obtained from GIBCO (Grand Island, NY). Poly-L-lysine, trypsin, soybean trypsin inhibitor, DNase, cytosine arabinoside, gentamycine sulphate, glutamine were obtained from Sigma Chemical Co.. Anti-MAP2 was purchased from Chemicon (Temecula, CA).
Results
Reproduction Data
An overall one-way ANOVA showed that dam weight gain, pregnancy length and litter size at birth were not significantly affected by prenatal exposure to WIN. In addition, exposure to WIN did not influence body weight and postnatal mortality of male pups (data not shown).
Neurochemical Experiments
Table 1 summarizes the numbers of analysed cell cultures, animals and pregnancies in each set of in vitro experiment.
Basal and KCl-evoked Extracellular Glutamate Levels
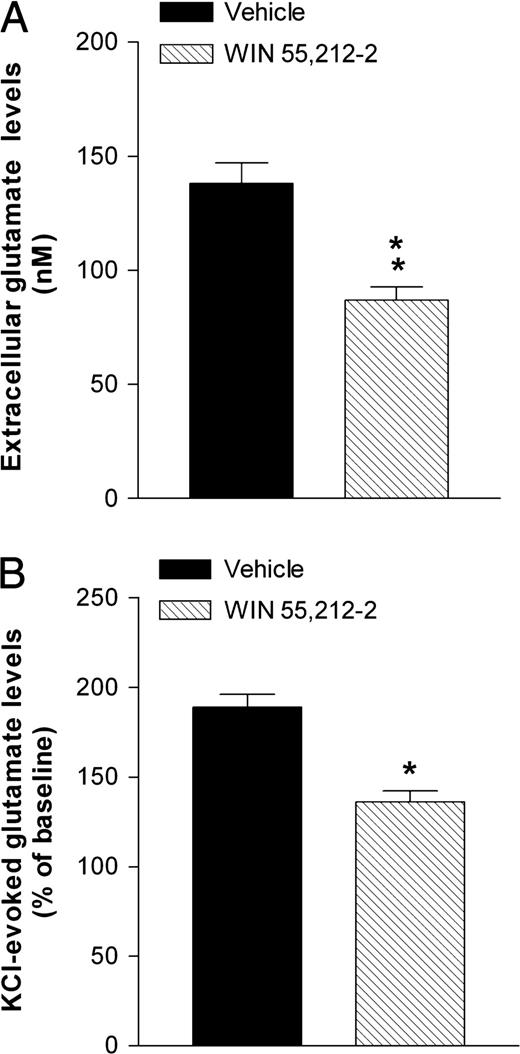
In the cortical cell cultures obtained from neonates born from mothers exposed to the vehicle, basal glutamate levels were (141 ± 9 nM; 11.28 ± 0.6 pmol/μg protein) and remained essentially stable over the duration of the experiment (i.e. five collected fractions; 150 min). Basal glutamate levels collected from the cortical cell cultures obtained from one-day old pups born from mothers exposed to WIN during pregnancy were significantly lower (87 ± 5 nM; 6.9 ± 0.3 pmol/μg protein) than that observed in cell cultures obtained from pups born from mothers exposed to the vehicle (Fig. 1A).
Effects of prenatal exposure to WIN 55,212-2 on basal (A) and K+-evoked (B) extracellular glutamate levels in cortical cell cultures obtained from 1-day-old pups. The WIN group represents the cortical cells obtained from neonates born from mothers treated daily with WIN 55,212-2 at a dose of 0.5 mg/kg s.c., from GD 5 to GD 20. WIN was suspended in 0.3% Tween 80–saline. The vehicle group represents the cortical cells obtained from neonates born from mothers treated with the 0.3% Tween 80–saline solution during gestation. Each value represents the mean ± SEM. *P < 0.05, **P < 0.01 significantly different from the respective vehicle group (Student's t-test).
As shown in Figure 1B, when a 20 min pulse of high K+ (20 mM) was used as depolarizing stimulus, a significant increase of glutamate efflux in both groups of cell cultures was found. However, the K+-evoked glutamate efflux in cortical cell cultures from rats born from mothers exposed during pregnancy to WIN was significantly lower than that observed in rats born from mothers exposed to the vehicle. No significant difference was found both in cortical basal and K+-evoked glutamate efflux between cell cultures of pups born from untreated mothers and those obtained from mothers exposed to the vehicle (data not shown).
Effect of NMDA on Basal Extracellular Glutamate Levels
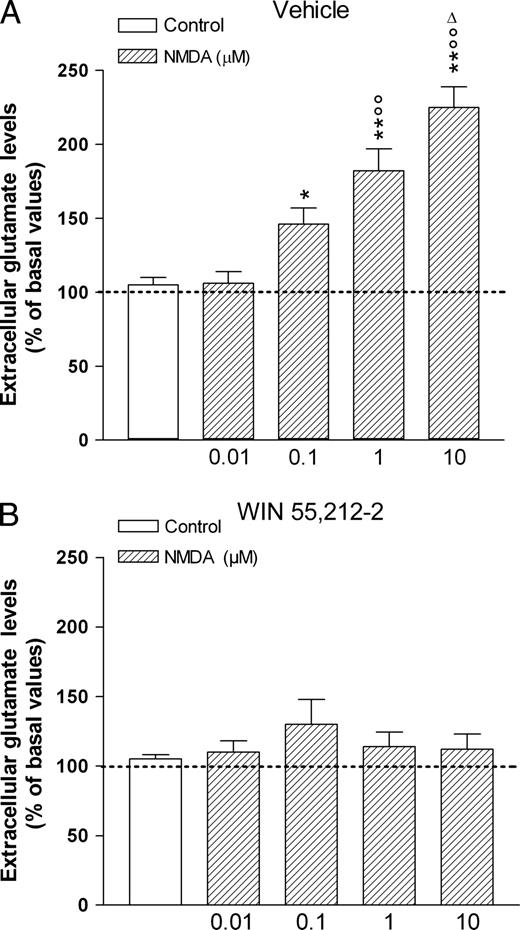
To evaluate whether the prenatal exposure with the CB1 receptor agonist affects cortical NMDA receptor function, we investigated the effects of locally applied NMDA on the endogenous glutamate extracellular levels.
In cortical cell cultures from one day old neonates born from mothers exposed to the vehicle during pregnancy, NMDA (0.01–10 μM) concentration-dependently increased extracellular glutamate levels (Fig. 2A). In contrast, NMDA in the same concentration range (0.01–10 μM) did not significantly affect extracellular glutamate levels in cortical cell cultures from pups born from mothers exposed during pregnancy to WIN (Fig. 2B).
Effect of NMDA on the extracellular glutamate levels in cortical cell cultures obtained from 1-day-old neonates. The WIN group (B) represents the cortical cells obtained from neonates born from mothers treated daily with WIN 55,212-2 at a dose of 0.5 mg/kg s.c., from GD 5 to GD 20. WIN was suspended in 0.3% Tween 80–saline. The vehicle group (A) represents the cortical cells obtained from neonates born from mothers treated with the 0.3% Tween 80–saline solution during gestation. Glutamate levels were measured in the third (30 min) fraction and are expressed as percent change of the basal values as calculated by the mean of the first two fractions. NMDA was added to the Krebs' Ringer solution 15 min before the end of the third fraction. Each value represents the mean ± SEM. *P < 0.05, **P < 0.01 significantly different from the respective control and 0.01 μM NMDA groups; °°P < 0.01 significantly different from the respective 0.1 μM NMDA group; ΔP < 0.01 significantly different from the respective 1 μM NMDA group according to ANOVA followed by Newman–Keul's test for multiple comparisons.
Behavioural Experiments
Homing behaviour
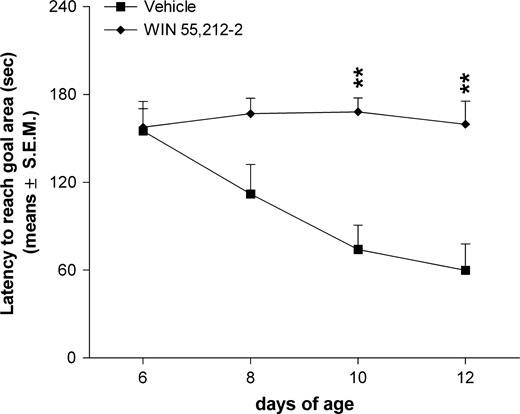
Two-way ANOVA for the latency to reach the goal area showed the following results: Ftreatments = 12.90, df = 1/20, P < 0.001; Fages = 5.63, df = 3/60, P < 0.001, Ftreatments×ages = 6.73, df = 3/60, P < 0.001. Post-hoc comparisons showed a significant increase (P < 0.01) of the latency to reach the goal area in WIN treated group as compared to the vehicle group on PNDs 10 and 12 (Fig. 3).
Effects of prenatal WIN 55,212-2 exposure on homing behaviour of offspring. The WIN group represents rats born from mothers treated daily with WIN 55,212-2 at a dose of 0.5 mg/kg s.c., from GD 5 to GD 20. WIN was suspended in 0.3% Tween 80–saline. The vehicle group represents rats born from mothers treated with the 0.3% Tween 80–saline solution during gestation. **P < 0.01 significantly different from the vehicle group (Tukey's test).
Ultrasonic Vocalization
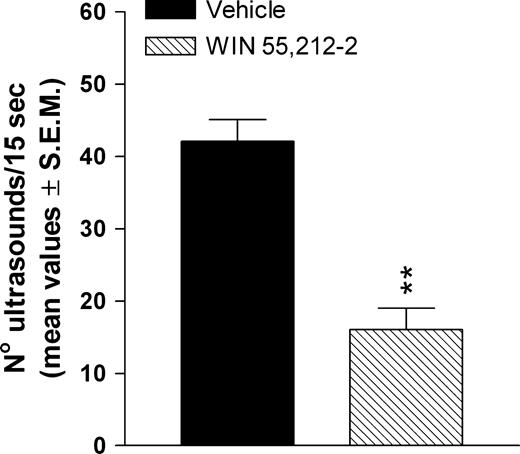
The data presented in Figure 4 show that prenatal treatment with WIN significantly decreases (P < 0.001, Student's t-test) the rate of calling (no. of ultrasounds/15 s) in 10-day-old pups removed from their nest.
Effects of prenatal WIN 55,212-2 exposure on ultrasonic vocalization of 10-day-old pups removed from their nest. The WIN group represents rats born from mothers treated daily with WIN 55,212-2 at a dose of 0.5 mg/kg s.c., from GD 5 to GD 20. WIN was suspended in 0.3% Tween 80–saline. The vehicle group represents rats born to mothers treated with the 0.3% Tween 80–saline solution during gestation. **P < 0.001 significantly different from the vehicle group (Student's t-test).
Active Avoidance Conditioning
Prenatal WIN exposure significantly impairs the acquisition of an active avoidance task. In particular, the present findings indicate that the percentage of rats (80 days old) achieving the criterion of 80% of CARs for three consecutive sessions was significantly lower (P < 0.02, Fisher's exact test) in WIN treated animals (30%) with respect to the vehicle group (90%).
Morphological Experiments
MAP2 Immunocytochemistry
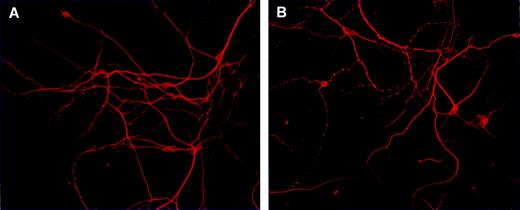
In view of the neurochemical and behavioural alterations observed in the offspring prenatally exposed to WIN, and knowing the role of glutamate in the development of the mammalian brain, a further set of morphological experiment was designed in order to preliminarily evaluate whether prenatal exposure to WIN could affect processes such as neuronal proliferation and development. Cortical cell cultures obtained from similar experimental conditions as used for neurochemical experiments were stained with an antibody for the neuronal marker MAP2, which is widely used to identify neurons in culture (Banker and Goslin, 1991). The cortical cell cultures obtained from pups born from vehicle-exposed mothers showed a high number of healthy neurons, which developed in a monolayer to form a complex network of neurites (see Fig. 5A for a representation). A different growth pattern was observed in the cortical cultures obtained from pups born from mothers exposed during pregnancy to WIN, where a minor population of neurons was present (see Fig. 5B for a representation). Interestingly, staining of the neurites with MAP2 antibody revealed an abnormal neurite outgrowth, characterized by the impairment of neurites branching. Indeed neurites appear more thin and shorter than those in the control cultures. A different distribution of MAP2 immunoreactivity along the neurites was observed, since MAP2 immunoreactivity appears condensed in some areas of the neurite, while it disappears completely in other areas (see Fig. 5B for a representation).
Representative photomicrographs of MAP2 immunocytochemistry in primary cortical neurons obtained from 1-day-old pups born from vehicle- (A) and WIN-exposed dams during pregnancy (B). Surviving neurons were stained with anti-MAP-2 antibody and observed under a fluorescent microscope.
Discussion
The results of the present study provide new evidence that the exposure during pregnancy to the CB1 receptor agonist WIN, at a dose that is not associated with overt signs of toxicity, causes an impairment in cortical glutamatergic neurotransmission and NMDA receptor function in the offspring. These neurochemical alterations are paralleled by learning disruption and changes in emotional reactivity. In particular, basal extracellular glutamate levels measured in cortical cell cultures obtained from neonates prenatally exposed to WIN are markedly reduced in comparison to those measured in cortical cell cultures obtained from neonates born from vehicle-exposed dams. The impairment in cortical glutamatergic neurotransmission is still evident in presence of an activation of cortical cells induced by a chemical depolarizing stimulus. In fact, the KCl-evoked glutamate efflux from cortical cells obtained from one-day old neonates born from mothers exposed to WIN during pregnancy is compromised, since in these neurons the enhancement of glutamate levels induced by the depolarizing stimulus is less pronounced. Thus, evidence is provided that the chronic activation of CB1 receptor during pregnancy impairs cortical glutamatergic neurotransmission in the offspring. It is worth noting that a similar reduction in glutamate release was also observed in hippocampal cell cultures obtained from pups born from mothers exposed to WIN as well as in the hippocampus and cerebral cortex of juvenile and adult rats born from WIN-treated dams (Mereu et al., 2003; Antonelli et al., 2004). The demonstration that prenatal exposure to WIN induced a reduction in the number of cortical neuronal population and disturbances in neurite outgrowth characterized by an impairment in neurites branching could suggest that these alterations might represent one of the possible mechanisms underlying the reduction in glutamate levels. In view of these findings and considering the important role of glutamate in neuronal proliferation (Luk et al., 2003), it could be speculated that prenatal exposure to WIN, by influencing the complexity of the basic mechanisms of neuronal proliferation and areal and laminar deployment (Ang et al., 2003), might lead to an inappropriate neuronal development that could represent the substrate for the observed learning deficits and decreased emotional activity in the exposed offspring. Such a hypothesis is in line with recent findings indicating that minimal disturbances or even small mistakes in the developmentally ordered facets might alter local neuronal circuitry and/or brain architecture, leading to subtle but functionally significant disorders which might underlie serious neurological disorders (Kozloski et al., 2001; Ang et al., 2003). In this context the misplacement or deficits of GABAergic cells have been postulated to be involved in a variety of developmental disorders such as in cortical dysplasia causing epilepsy (Roper et al., 1999), autism (Casanova et al., 2002) and Tourette's syndrome (Leckman and Riddle, 2000).
The alteration in cortical glutamatergic neurotransmission and neuronal population, observed in the present study, is associated with learning deficit and decreased emotional reactivity. In particular, rat pups (PNDs 10–12) exposed to WIN during gestation, exhibited a profound impairment of the acquisition of homing behaviour, which is a simple form of learning during the early phases of postnatal life. This test exploits the strong tendency of the immature neonate to maintain body contact with its mother and siblings. This behaviour requires intact sensory, olfactory, motor and ultrasonic capabilities, as well as adequate associative and discriminative capabilities that allow the pup to become imprinted by the mother's odour, and to memorize and recognize it among others (Bignami, 1996).
A learning impairment was still present in adult offspring (PND 80) prenatally exposed to WIN and tested in an active avoidance task. The learning disruption does not appear to be attributable to alterations of a non-associative nature. In fact, the learning deficit produced by prenatal WIN may be dissociated from the hyperactivity, which has also been reported to be found postnatally by prenatal WIN exposure (Mereu et al., 2003). In fact, behavioural hyperactivity was observed at PND 12 but not PND 80, whereas the learning deficit was observed at both time periods.
Taken together these findings indicate that the gestational exposure to WIN induces a significant impairment of the glutamatergic neurotransmission both in the cortex (present study) and the hippocampus (Mereu et al., 2003), two brain regions which are crucial for certain forms of learning and memory. In this context, the local neuronal network of the hippocampus, as well as the hippocampus–frontal cortex pathway, shows short- and long-term synaptic plasticity, such as paired-pulse facilitation and long-term potentiation (LTP), which are thought to be the neural basis for learning and memory. Thus, a reduction in cortical and hippocampal glutamatergic neurotransmission may represent a neurochemical substrate for the reported disruption in LTP which might in turn underlie, at least in part, the long-lasting impairment in cognitive function caused by the gestational exposure to the cannabinoid receptor agonist (Kalant, 2004; Smith et al., 2004).
The behavioural results of the present study also show that prenatal WIN exposure decreases the rate of ultrasonic emission in 10-day-old pups. The reduced rate of calling produced by prenatal WIN exposure reflects a decrease in the emotional reactivity of offspring. Indeed, the rate of calling (as determined by the number of ultrasonic calls per time unit) is a reliable indicator of the emotional state of the rat. Moreover, the measurement of ultrasonic vocalizations provides a valuable tool for detecting changes in emotional reactivity produced by adverse developmental treatments with a potential for extrapolation to humans. In fact, ultrasonic emissions in rodents have communication purposes, and it has been suggested that this function may be related to human infant crying (Elsner et al., 1990). In this regard, several developmental studies in humans have shown that particular changes in the acoustic features of the neonatal cry seems to be an early indicator of long-term alterations in the neurophysiological status caused by adverse pre- and postnatal events (Lester, 1987; Michelsson et al., 1997). Furthermore, because alterations in rat pup ultrasonic calling influence maternal behaviour which, in turn, might affect the behaviour of the offspring, the changes in ultrasonic vocalization elicited by prenatal exposure to WIN could have a role in the development of behavioural abnormalities observed in the adult animals.
Concerning the clinical relevance of the present study, it is important to estimate, by extrapolation, if the dose of WIN administered compares with that of Δ9-THC absorbed by cannabis users. Previous studies have estimated that a 5 mg/kg dose of Δ9-THC in rats corresponds to a moderate exposure of the drug in humans, correcting for the differences in route of administration and the ration of body weight to surface area (Garcia-Gil et al., 1997, 1998). However, depending on the administration route and the endpoints considered the CB1 receptor agonist WIN is 3–10 times more potent than Δ9-THC (French et al., 1997; Hampson and Deadwyler, 2000). This estimate is consistent with the relative Ki of each compound for the CB1 receptor in neuronal membranes (i.e. 2–12 nM compared with 35–80 nM, for WIN and Δ9-THC respectively; Pertwee, 2000). Based on these considerations, the dose of WIN employed in the present study corresponds to a low-to-moderate exposure to cannabis in human.
Another relevant finding of this study is the demonstration that prenatal exposure to WIN induces a significant loss of the NMDA receptor-mediated regulation of glutamate levels. In fact, the concentration-dependent increase of glutamate levels observed in cell cultures obtained from neonates born from vehicle-treated dams is completely lost in neuronal cell cultures obtained from pups prenatally exposed to WIN. Previous current-clamp studies indicate that acute WIN administration did not alter the membrane response to NMDA or passive membrane properties of granule neurons suggesting that NMDA receptors may not be the primary sites of cannabinoid receptor action (Netzeband et al., 1999). In view of these differences, the present results open up a new scenario suggesting that chronic prenatal treatment with WIN induces a loss of NMDA receptor activity in the offspring. However, at the present, the mechanisms underlying neurochemical interaction and/or signalling pathways between CB1 and NMDA receptors observed in the present study remain to be elucidated. In this context, it is worth noting that in cerebellar granule neurons, cannabinoids modulate NMDA-mediated signals by interfering with calcium release from IP3-gated stores and this effects involves a phospholipase C-sensitive mechanisms (Netzeband et al., 1999). Since the involvement of IP3-mediated mechanism has been also demonstrated for the WIN-induced effect on glutamate release (Ferraro et al., 2001), it could be speculated that prenatal WIN treatment induced an alteration of NMDA receptor signals through a post-junctional phosphorylation mechanism, by involving, inter alia, phospholipase C. However, this hypothesis remains to be demonstrated in a further study and other mechanism(s) cannot be excluded. Previous studies performed in hippocampus and striatal slices have demonstrated that CB1 receptor activation at micromolar concentrations inhibits NMDA- and kainate-stimulated noradrenaline release in guinea-pig hippocampus as well as NMDA-stimulated dopamine release in rat striatum (Kathmann et al., 1999). In addition, Hampson et al. (1998) have described an inhibitory modulation of the NMDA-elicited signal, which is mediated by CB1 receptors in cerebellar and cortical slices. The ability of the CB1 receptor to regulate NMDA receptor-mediated activity has been further demonstrated in two recent studies. Parmentier-Batteur et al. (2002) demonstrated that the absence of the CB1 receptor exacerbates the excitotoxicity of directly applied NMDA in CB1 knockout mice. This finding was explained by the fact that neuronal CB1 receptors are thought to reside primarily on presynaptic nerve terminals, where their activation inhibits voltage-gated calcium channels. Furthermore, their neuroprotective effect in ischaemia could be mediated partly through the inhibition of depolarization-induced glutamate release (Shen et al., 1996). How the absence of CB1 receptors exacerbates the excitotoxicity of directly applied NMDA is unclear, but the more severe NMDA-induced injury in CB1 knockout mice suggests that endogenous cannabinoid signalling can also regulate excitotoxicity indirectly by recruiting other neurotransmitter systems. In a second study, intra-periaquaductal grey (PAG) microinjection of WIN dose-dependently increased the latency of the nociceptive reaction in the plantar test (Palazzo et al., 2001). Furthermore, pretreatment with a selective NMDA receptor antagonist DL-AP5 blocked the effect of WIN. This study suggests that the physiological stimulation of mGlu and NMDA receptors in the PAG is required for the cannabinoid-induced analgesia in this midbrain area. Because CB1 receptor activation is coupled to a variety of effectors, including ion channels, adenylate cyclase and protein kinases (Pertwee, 1997), there are numerous signalling pathways through which such an effect might occur. Taken together, these findings suggest the existence of a neurochemical interaction between CB1 and NMDA receptors. In particular, the findings presented in this study indicate that a prolonged prenatal stimulation of the CB1 receptor could lead to a reduced responsiveness of cortical NMDA receptor.
In view of the above, it is also possible that the inhibitory effects mediated by cortical CB1 receptor activation might be mediated by a reduced presynaptic glutamate release induced by a prevention of NMDA-mediated mechanisms. In this context, it may also be hypothesized that the disruption in hippocampal LTP observed in offspring of dams exposed to WIN during pregnancy (Mereu et al., 2003) might be derived from an impairment in NMDA receptor activity and a reduction in glutamatergic neurotransmission. From a functional point of view, it is well known that the biochemical events underlying learning and memory require the involvement of glutamatergic neurotransmission. In support of this statement, NMDA receptor antagonists alter acquisition and retention in various learning tasks, suggesting a mediation by NMDA receptors, which in turn appears to be closely linked to the role of these receptors in synaptic plasticity of the central nervous system (Bliss and Collingridge, 1993). In addition, since (i) anxiolytic drugs selectively reduce the rate of ultrasonic calling, whereas anxiogenic agents increase the number of calls/time unit (Insel et al., 1986), and (ii) NMDA receptor antagonists also induce anxiolytic effects, as measured by separation-induced ultrasonic vocalizations (Kehne et al., 1991), the decrease in ultrasonic vocalization caused by prenatal WIN exposure could be partly attributable to an impairment in NMDA receptor function induced by exposure to the CB1 receptor agonist in utero.
In view of the above, and considering that the NMDA receptors play an early role in the regulation of calcium-dependent cell migration before neurons reach their targets and form synaptic contacts (Komuro and Rakic, 1993), it could be suggested that prenatal exposure to WIN by altering the functional activity of glutamatergic neurotransmission and of the NMDA receptors could induce a disturbance in neurite outgrowth and an alteration in neuronal development which could result in an impairment in learning and a reduction in emotional reactivity in the offspring. This stimulant hypothesis will be developed in further studies.
This study was supported by Ministero dell'Istruzione, dell'Università e della Ricerca (Progetti di Ricerca di Rilevanza Nazionale and Fondo per gli Investimenti della Ricerca di Base 2002). The authors thank Fondazione Cassa di Risparmio di Ferrara, Italy.
References
Alho H, Ferrarese C, Vicini S, Vaccarino F (
Aggleton JP, Neave N, Nagle S, Sahgal A (
Ang ESBC, Haydar TF, Gluncic V, Rakic P (
Annau Z, Cuomo V (
Antonelli T, Tanganelli S, Tomasini MC, Finetti S, Trabace L, Steardo L, Sabino V, Carratu MR, Cuomo V, Ferraro L (
Auclair N, Otani S, Soubrie P, Crepel F (
Bignami G (
Bliss TV, Collingridge GL (
Bradford M (
Cagiano R, Barfield RJ, White NR, Pleim ET, Weinstein M and Cuomo V (
Cagiano R, Cassano T, Coluccia A, Gaetani S, Giustino A, Steardo L, Tattoli M, Trabace L, Cuomo V (
Casanova MF, Buxhoeveden DP, Switala AE, Roy E (
Dalterio SL (
Day NL, Richardson GA, Geva D, Robles N (
De Salvia MA, Cagiano R, Carratù MR, Di Giovanni, Trabace L, Cuomo V (
Elsner J, Suter D, Alder S (
Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T (
French ED, Dillon K, Wu X (
Fried PA, Smith AM (
Garcia-Gil L, De Miguel R, Munoz RM, Cebeira M, Villanua MA, Ramos JA, Fernandez-Ruiz JJ (
Garcia-Gil L, Ramos JA, Rubino T, Parolaro D, Fernandez-Ruiz JJ (
Hampson AJ, Bornheim LM, Scanziani M, Yost CS, Gray AT, Hansen BM, Leonoudakis DJ, Bickler PE (
Hampson RE, Deadwyler SA (
Hernandez ML, Garcia-Gil L, Berrendero F, Ramos JA, Fernandez-Ruiz JJ (
Insel TR, Hill JL, Mayor RB (
Kalant H (
Kathmann M, Bauer U, Schlicker E, Gothert M (
Kehne JH, McCloskey TC, Baron BM, Chi EM, Harrison BL, Whitten JP, Palfreyman MG (
Kozloski J, Hamzei-Sichani F, Yuste R (
Leckman JF, Riddle MA (
Lester BM. (
Lichtman AH, Martin BR (
Luk KC, Kennady TE, Sadikot AF (
Mereu G, Fa M, Ferraro L, Cagiano R, Antonelli T, Tattoli M, Ghiglieri V, Tanganelli S, Gessa GL, Cuomo V (
Michelsson K, Sirvio P, Wasz-Hockert O (
Misner DL, Sullivan JM (
Navarro M, Rubio P, Rodriguez De Fonseca F (
Netzeband JG, Conroy SM, Parsons KL, Gruol DL (
Palazzo E, Marabese I, de Novellis V, Oliva P, Rossi F, Berrino L, Rossi F, Maione S (
Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA (
Paule MG, Bushnell PJ, Maurissen JP, Wenger GR, Buccafusco JJ, Chelonis JJ, Elliott R (
Pertwee RG (
Ragozzino ME, Adams S, Kesner RP (
Roper SN, Eisenschenk S, King MA (
Rubio P, Rodriguez de Fonseca F, Martin-Calderon JL, Del Arco I, Bartolome S, Villanua MA, Navarro M. (
Shen M, Piser TM, Seybold VS, Thayer SA (
Smith AM, Fried PA, Hogan MJ, Cameron J (
Stella N, Schweitzer P, Piomelli D (
Sullivan JM (
Terranova JP, Michaud JC, Le Fur G, Soubrie P (
Vela G, Martin S, Garcia-Gil L, Crespo JA, Ruiz-Gayo M, Javier Fernandez-Ruiz J, Garcia-Lecumberri C, Pelaprat D, Fuentes JA, Ramos JA, Ambrosio E (
Author notes
1Department of Clinical and Experimental Medicine, Pharmacology Section, University of Ferrara, Italy, 2Department of Pharmacology and Human Physiology, University of Bari, Italy, 3Department of Biomedical Sciences, University of Foggia, Italy, 4Institute of Pharmacology and Pharmacognosy, University of Palermo, Italy and 5Department of Pharmacology and General Physiology, University ‘La Sapienza’ Roma, Italy