-
PDF
- Split View
-
Views
-
Cite
Cite
N.V. Povysheva, G. Gonzalez-Burgos, A.V. Zaitsev, S. Kröner, G. Barrionuevo, D.A. Lewis, L.S. Krimer, Properties of Excitatory Synaptic Responses in Fast-spiking Interneurons and Pyramidal Cells from Monkey and Rat Prefrontal Cortex, Cerebral Cortex, Volume 16, Issue 4, April 2006, Pages 541–552, https://doi.org/10.1093/cercor/bhj002
Close - Share Icon Share
Abstract
In the prefrontal cortex (PFC) during working memory tasks fast-spiking (FS) interneurons might shape the spatial selectivity of pyramidal cell firing. In order to provide time control of pyramidal cell activity, incoming excitatory inputs should excite FS interneurons more vigorously than pyramidal cells. This can be achieved if subthreshold excitatory responses of interneurons are considerably stronger and faster than those in pyramidal neurons. Here we compared the functional properties of excitatory post-synaptic potentials (EPSPs) between pyramidal cells and FS interneurons in slices from monkey dorsolateral PFC and rat prelimbic cortex. Miniature, unitary (in connected pairs or by minimal stimulation) and compound (evoked by electrical stimulation of the white matter) EPSPs were recorded in whole cell mode. We found that EPSPs were significantly larger and faster in FS interneurons than those recorded from pyramidal cells, consistent with the idea of more efficient recruitment of FS interneurons compared to pyramidal neurons. Similar results were obtained in monkey and rat PFC, suggesting a stable role of FS interneurons in this circuitry across species.
Introduction
The prefrontal cortex (PFC) is one of the most expanded and differentiated regions of the brain in primates and humans relative to non-primate species. It is involved in a number of complex behaviors and various aspects of cognition, including executive functions and working memory (Goldman-Rakic, 1995, 1996; Miller and Cohen, 2001; Baddeley, 2003). To date, working memory is one of the most studied functions of the PFC, although the precise cellular basis of working memory processes remains elusive.
In vivo recordings from monkey dorsolateral PFC during spatial working memory tasks have demonstrated the selective activation of certain neurons by specific locations of a visual cue (Funahashi et al., 1989). Both regular-spiking pyramidal cells and fast-spiking (FS) interneurons appear to be tuned to specific locations during all phases of spatial working memory tasks (Wilson et al., 1994; Rao et al., 1999, 2000; Constantinidis and Goldman-Rakic, 2002). Inhibition provided by FS interneurons has been proposed to sharpen spatial selectivity of pyramidal cells, since blockade of GABAA receptors result in expansion of the neuronal receptive field of dorsolateral PFC neurons (Rao et al., 2000). The important contribution of FS interneurons in shaping spatial tuning of pyramidal cells has been also shown in modeling studies (Tanaka, 1999; Wang et al., 2004).
In order to sharpen the spatial tuning of neighboring pyramidal cells, FS interneurons need to be more effectively recruited by excitatory inputs so that they can restrict the temporal summation of excitatory responses in their pyramidal cell targets and increase the temporal precision of their firing (Fricker and Miles, 2000). Indeed, data from in vivo studies show that in response to incoming excitation, FS neurons in rat and rabbit somatosensory cortex fire more vigorously, more reliably and at shorter latencies than do regular spiking pyramidal cells (Simons, 1978; Simons and Carvell, 1989; Swadlow, 1989). Such excitation of interneurons that is more effective and more temporally precise than that of pyramidal cells could result from specific properties of the subthreshold excitatory responses. Specifically, excitatory post-synaptic potentials (EPSPs) with larger amplitude and faster time course would lower the threshold and shorten the latency for EPSP–spike coupling in interneurons compared to pyramidal cells (Fricker and Miles, 2000; Galarreta and Hestrin, 2001; Maccaferri and Dingledine, 2002).
Some previous studies of rodent sensory systems have demonstrated differential properties of single-axon excitatory responses in FS interneurons and pyramidal cells (Thomson, 1997; Beierlein et al., 2003). However, the extrapolation of these findings to the primate PFC that subserves complex cognitive tasks distinctive to primates is limited, because other aspects of GABA neurons do not generalize well from rodents to primates. For example, cortical interneurons have different developmental origins in rodents and primates (Letinic et al., 2002; Xu et al., 2004). Furthermore, rodents and primates differ considerably in the proportions of neocortical interneurons immunoreactive for specific calcium-binding proteins: parvalbumin-positive interneurons are the most prevalent interneuron subpopulation in rat PFC (Gabbott et al., 1997; Kawaguchi and Kubota, 1997), whereas calretinin-positive interneurons dominate in the PFC of monkeys (Conde et al., 1994; Gabbott and Bacon, 1996). Such species differences raise the question of whether the inhibitory mechanisms observed in primates are the same as those in rats.
Thus, in this study we examined the properties of EPSPs in FS interneurons and pyramidal cells of monkey and rat PFC to determine if the subthreshold excitatory responses had differential properties consistent with the previous observations of more efficient recruitment of FS interneurons compared to pyramidal neurons. We have determined the physiological properties of miniature EPSPs (mEPSPs), unitary EPSPs (uEPSPs; in connected pairs or by minimal stimulation), and EPSPs evoked by electrical stimulation of the white matter (eEPSPs).
Materials and Methods
Slice Preparation
Young male Wistar rats (P19–P29) and young adult (3.5–6.0 kg) male cynomolgus monkeys (Macaca fascicularis) were treated in accordance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Rats were deeply anesthetized with halothane and decapitated. The brain was quickly removed and immersed in ice-cold pre-oxygenated artificial cerebrospinal fluid (ACSF). The protocol employed to obtain tissue blocks from monkey PFC has previously been described in detail (Gonzalez-Burgos et al., 2000, 2004). Briefly, animals were treated with ketamine hydrochloride (25 mg/kg, i.m.), dexamethasone phosphate (0.5 mg/kg, i.m.), and atropine sulfate (0.05 mg/kg, s.c.); an endotracheal tube was inserted and anesthesia was maintained with 1% halothane in 28% O2/air. Monkeys were placed in a stereotaxic apparatus and a craniectomy was performed over the doroslateral PFC. The dura was removed in a location determined by stereotaxic coordinates and by the position of relevant sulcal landmarks, and a small block of tissue was excised containing a portion of dorsal area 9 and both the medial and lateral banks of the principal sulcus (area 46). After the surgery, the animals were treated with an antibiotic (chloramphenicol, 15 mg/kg, i.m.) and an analgesic (hydromorphone, 0.02 mg/kg, i.m.) three times a day. All animals recovered quickly with no impairments in eating or drinking or overt behavioral deficits. In most cases, the animals underwent the same procedure 2–4 weeks later to obtain tissue from a non-homotopic region of the opposite hemisphere. During the second procedure, after the craniectomy, the animal was given an overdose of pentobarbital (30 mg/kg) and was perfused through the heart with ice-cold modified ACSF. A tissue block containing portions of areas 9 and 46 nonhomotopic to the first biopsy was quickly excised. Subsequent treatment of the tissue was the same for both days.
Tissue blocks containing the prelimbic cortex in rats (Paxinos and Watson, 1998) or areas 46 (both ventral and dorsal banks) and 9 (dorsal) of monkey PFC were placed in ice-cold ACSF, and 350 μm thick coronal slices were cut with a vibratome (Leica VT1000S, Leica, Germany). Slices were incubated at 37°C for 1 h and at room temperature thereafter. Slices were transferred to a recording chamber and perfused with ACSF at 31–32°C. Through all steps of the experiments, ACSF of the following composition (in mM) was used: 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 24 NaHCO3, 10 dextrose and perfused with 95% O2/5% CO2 gas mixture. Tissue slices from the same monkeys were also used in other studies (Gonzalez-Burgos et al., 2004, 2005; Zaitsev et al., 2005).
Electrophysiological Recordings
Whole-cell recordings were made from layer 2–3 neurons visually identified using IR-DIC videomicroscopy on a Zeiss Axioskop 2 FS microscope with a 40× water immersion objective and a Dage-MTI NC-70 video camera (Dage-MTI Television, Michigan City, IN). Pyramidal cells were selected based on their relatively large triangular soma and visible apical dendrite. Interneurons typically had smaller somata, which were round or oval in shape. Glass micropipettes were filled with an internal solution containing (in mM): 114 potassium gluconate, 6 KCl, 10 HEPES, 4 ATP-Mg and 0.3 GTP. The pH was adjusted to 7.25 with KOH. Biocytin (0.5%; Molecular Probes, Eugene, OR) and Alexa 488 or 568 (0.075%; Molecular Probes) were added to the solution for later morphological identification of the recorded neurons. In some experiments QX-314 (5 mM; Sigma, St Louis, MO) was added to the intracellular solution. Electrodes had 9–12 MΩ open-tip resistance in current-clamp recordings, whereas 5–6 MΩ electrodes were used in voltage clamp experiments. Voltages were amplified with IE-210 electrometers (Warner Instruments, Hamden, CT) operating in bridge-balance mode. Currents were recorded with a MultiClamp amplifier (Axon Instruments, Union City, CA). Signals were filtered at 5, or 4 KHz in the IE-210 and the MultiClamp, respectively, and acquired at a sampling rate of 20 kHz using a 16 bit-resolution Power 1401 interface and Signal 2 software (CED, Cambridge, UK). Access resistance and capacitance were compensated on-line. Access resistance typically was 15–30 MΩ and remained relatively stable during experiments (≤30% increase) for the cells included in the analysis.
Electrophysiological Data Analysis
Intrinsic Membrane Properties
To characterize the membrane properties of neurons, hyper- and depolarizing rectangular pulses of 500 ms duration were applied in 5–10 pA increments at 0.5 Hz. Amplitude of the action potential (AP) and afterhyperpolarization (AHP) were measured from the threshold of the first AP. Duration of the AP was measured at its half amplitude. To evaluate the spike frequency adaptation in evoked trains, an adaptation ratio (A-ratio) was calculated as the ratio between the duration of the first and last inter-spike interval at a stimulation level of 60 pA above firing threshold. Input resistance was measured from the slope of a linear regression fit to the voltage–current relation in the hyperpolarizing range. The membrane time constant was determined by single-exponential fitting to the on-phase of the average voltage responses to hyperpolarizing current steps of −5 to −20 pA.
Miniature Responses
mEPSPs were recorded at a membrane potential of −74 ± 4 mV in both rat and monkey slice neurons. Miniature excitatory post-synaptic currents (mEPSCs) were recorded at a holding membrane potential of −80mV. Tetrodotoxin (TTX; 0.5 μM; Sigma) and bicuculline methiodide (BMI; 10–20 μM; Sigma) were added to the bath to block APs and GABAA-mediated inhibitory postsynaptic potentials respectively.
Miniature events were analyzed using MiniAnalysis (Synaptosoft, Decatur, GA). Peak events were first detected automatically using an amplitude threshold of two times the average RMS noise, which was ∼0.3 mV for mEPSPs and ∼7 pA for mEPSCs. The relatively high detection threshold for miniature responses may have resulted in some events going undetected. Therefore, the mean amplitude of the analyzed mEPSCs might represent an overestimation, while the frequency could be underestimated. After automatic analysis, events were rechecked by visual inspection of the traces and were accepted for analysis if they had a monophasic rising phase and decayed to baseline in an exponential manner. Between 100 and 300 events in each cell were included in the analysis. Amplitudes of miniature responses were determined from baseline to peak. The time constants of single exponential fits were used to describe the decay time. The rise time was estimated as the time necessary to rise between 10 and 90% of the peak response.
Unitary Responses
Simultaneous recordings from 2–6 neurons were used to study unitary synaptic responses between connected pairs of cells. Square current pulses sufficient to elicit an AP were injected to presynaptic neuron at a frequency of 0.1 Hz. uEPSPs were recorded at a resting membrane potential of −72 ± 5 mV. Unitary inhibitory post-synaptic potentials (uIPSPs) were recorded at a depolarized membrane potential of −54 ± 4 mV. Average responses were generated from 21–110 individual traces including failures. Amplitude and time course were measured on averaged sweeps. The latency of uIPSPs was measured between AP peak in FS interneurons and the onset of IPSP.
Responses Evoked by Extracellular Stimulation
Bipolar theta-glass electrodes (outside diameter 1.5 mm and tip diameter 2–5 μm) were placed on the border of white matter and layer VI in vertical register with the neurons recorded in layers 2–3. Currents were generated using A360 Stimulus Isolators (World Precision Instruments, Sarasota, FL) and were triggered digitally via the Power 1401 and Signal software. Synaptic responses were evoked by applying current steps of 0.2 ms duration, 0.1 Hz frequency and 80–200 μA current intensity. eEPSPs were recorded simultaneously in pairs of two pyramidal cells, a pyramidal cell and FS interneuron at a membrane potential of −72 ± 4 mV. To evoke synaptic responses we first applied a stimulation current of ∼200 μA, and then decreased its intensity to a level of 70–100 μA (30–60 μA above threshold) where we could obtain visible subthreshold responses in both cells with few failures (up to 10%) in both cells. Synaptic responses were included in the analysis if the rising phase was monotonic, and the jitter in latency was <1 ms. During these experiments 10–20 μM BMI was added to the bath to block inhibitory responses. In some experiments, D-2-amino-5-phospho-pentanoic acid (AP5; 50 μM; Sigma) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM; Sigma) were included in the bath to block N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors respectively. Amplitude, 10–90% rise time and decay time of the eEPSPs were measured on the averaged traces consisting of 15–30 repetitions, excluding failures. The time constant of a single exponential fit was used to describe decay time. Failures were defined as those events in which the EPSP amplitude was below the threshold of 2 times peak-to-peak noise.
Responses Obtained by Minimal Stimulation
Minimal stimulation-evoked responses were obtained similarly to eEPSPs, but with low intensities of stimulation current. More specifically, to evoke EPSPs by minimal stimulation, the stimulation current intensity was set close to the threshold to evoke an excitatory response. In some cells, an increase in the stimulation current within a range of ± 5% resulted in neither changes in response amplitude nor in failure rate (which was 40–60% in different cells). This stimulation range was considered as the range that evoked minimal responses, possibly due to stimulation of a single axon. Above this stimulus range, responses showed a significant increase in amplitude and a decrease in failure rate (up to 0%). However, due to the detection level for the smallest events that was possible to obtain in this study (see ‘Miniature responses’) the failure rate might be overestimated and the amplitude could be underestimated.
Statistical Analysis
t-Tests for independent and dependent samples were used for group comparisons. The Pearson (r) coefficient was used to estimate the significance of linear correlations. Comparison of cumulative distributions was made using Kolmogorov–Smirnov test (K-S Z). Values are presented as mean ± SD.
Morphological Analysis
During recordings, neurons were filled with the fluorescent dyes Alexa 488 or 568 (0.075%; Molecular Probes) and with biocytin (0.5%; Molecular Probes) added to the internal solution. Cells were recorded for at least 30 min in order to ensure extensive cell labeling by the dyes. Slices were fixed in ice-cold 4% paraformaldehyde for at least 72 h, then transferred into an anti-freeze solution (ethylene glycol and glycerol in 0.1 M phosphate buffer), and stored at −80°C. Neurons were reconstructed three-dimensionally using an Olympus Fluoview BX61 confocal microscope (Olympus America Inc, Melville, NY) with FITC and CY3 filters. Images were acquired with Fluoview software (Olympus America Inc, Melville, NY) and further processed using Imaris (Bitplane INC, Saint Paul, MN) and Photoshop (Adobe Systems Inc., San Jose, CA) programs. In addition, some slices were processed for biocytin. The fixed slices were transferred to 0.1 M Na-phosphate buffer, serially resectioned at 50 μm, and processed for visualization of the biotin label, using the Vectastain Elite ABC kit (Vector Laboratories Inc., Burlingame, CA) peroxidase reaction and NiDAB chromogen.
Results
Physiological Membrane Properties and Morphological Features of Pyramidal Cells and FS Interneurons
In layers 2–3 of rat and monkey PFC, pyramidal cells and interneurons were identified visually under differential interference contrast videomicroscopy. Pyramidal cells had triangular somata and a clear apical dendrite, whereas interneurons were characterized by a round or oval cell body and the lack of a visible apical dendrite. In both rat and monkey PFC, FS interneurons (Kawaguchi, 1993, 1995; Gonzalez-Burgos et al., 2004) were identified by their fast APs with low amplitude and deep AHP (Fig. 1A,B, Table 1). FS interneurons also generated trains of spikes with essentially constant interspike intervals during 500 ms sweeps. Thus, these cells expressed very little, if any, spike frequency adaptation. FS interneurons from rat and monkey PFC had very similar physiological properties, with the exception of input resistance, which was higher in FS interneurons from monkey PFC.
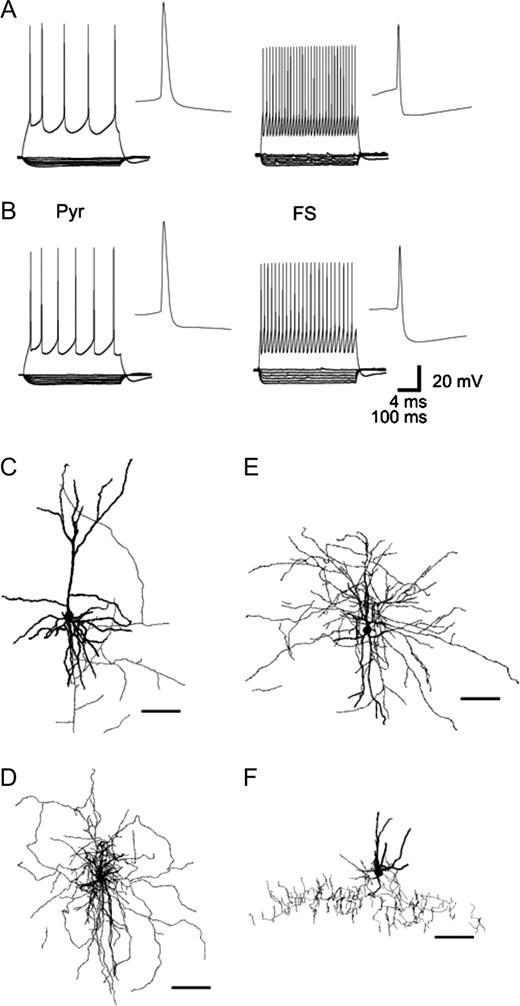
Electrophysiological and morphological properties of pyramidal cells and FS interneurons in rat and monkey PFC. In slices from both rat (A) and monkey PFC (B), depolarizing current injections (60 pA above spike threshold) evoked firing with prominent spike frequency adaptation in pyramidal cells (Pyr) but non-adapting firing in FS interneurons. APs in pyramidal cells had larger amplitude and longer duration than in FS interneurons. In pyramidal cells, the AHP is smaller than in FS interneurons. FS interneurons had significantly higher input resistance than pyramidal cells, as indicated by the response to hyperpolarizing current steps (10–50 pA). (C–F) Confocal microscope reconstructions of pyramidal cells and FS interneurons filled with fluorescent Alexa dyes during recording. In addition to a pyramidal cell (C), depicted are the morphological features of a local arbor cell (rat) (D), medium arbor cell (monkey) (E) and a chandelier neuron (rat) (F) (scale bar 100 μm).
Membrane properties of pyramidal cells and FS interneurons in rat and monkey PFC
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 12) . | Monkey (n = 14) . | Rat (n = 34) . | Monkey (n = 16) . | ||
| AP amplitude (mV) | 88 ± 8a | 86 ± 8a | 58 ± 14 | 56 ± 8 | ||
| AP duration (ms) | 0.95 ± 0.11a | 0.82 ± 0.11a | 0.44 ± 0.14 | 0.34 ± 0.08 | ||
| AHP amplitude (mV) | 16 ± 4a | 17 ± 2a | 26 ± 7 | 25 ± 6 | ||
| Adaptation ratio | 0.42 ± 0.25a | 0.51 ± 0.27a (n = 13) | 0.95 ± 0.22 (n = 31) | 0.94 ± 0.15 | ||
| Input resistance, (MΩ) | 135 ± 39 | 136 ± 55a | 161 ± 49 | 245 ± 78 | ||
| Time constant (ms) | 19 ± 6a | 18 ± 3a | 7 ± 2 | 9 ± 3 | ||
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 12) . | Monkey (n = 14) . | Rat (n = 34) . | Monkey (n = 16) . | ||
| AP amplitude (mV) | 88 ± 8a | 86 ± 8a | 58 ± 14 | 56 ± 8 | ||
| AP duration (ms) | 0.95 ± 0.11a | 0.82 ± 0.11a | 0.44 ± 0.14 | 0.34 ± 0.08 | ||
| AHP amplitude (mV) | 16 ± 4a | 17 ± 2a | 26 ± 7 | 25 ± 6 | ||
| Adaptation ratio | 0.42 ± 0.25a | 0.51 ± 0.27a (n = 13) | 0.95 ± 0.22 (n = 31) | 0.94 ± 0.15 | ||
| Input resistance, (MΩ) | 135 ± 39 | 136 ± 55a | 161 ± 49 | 245 ± 78 | ||
| Time constant (ms) | 19 ± 6a | 18 ± 3a | 7 ± 2 | 9 ± 3 | ||
Significantly different from FS interneurons within the same species (P < 0.05).
Membrane properties of pyramidal cells and FS interneurons in rat and monkey PFC
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 12) . | Monkey (n = 14) . | Rat (n = 34) . | Monkey (n = 16) . | ||
| AP amplitude (mV) | 88 ± 8a | 86 ± 8a | 58 ± 14 | 56 ± 8 | ||
| AP duration (ms) | 0.95 ± 0.11a | 0.82 ± 0.11a | 0.44 ± 0.14 | 0.34 ± 0.08 | ||
| AHP amplitude (mV) | 16 ± 4a | 17 ± 2a | 26 ± 7 | 25 ± 6 | ||
| Adaptation ratio | 0.42 ± 0.25a | 0.51 ± 0.27a (n = 13) | 0.95 ± 0.22 (n = 31) | 0.94 ± 0.15 | ||
| Input resistance, (MΩ) | 135 ± 39 | 136 ± 55a | 161 ± 49 | 245 ± 78 | ||
| Time constant (ms) | 19 ± 6a | 18 ± 3a | 7 ± 2 | 9 ± 3 | ||
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 12) . | Monkey (n = 14) . | Rat (n = 34) . | Monkey (n = 16) . | ||
| AP amplitude (mV) | 88 ± 8a | 86 ± 8a | 58 ± 14 | 56 ± 8 | ||
| AP duration (ms) | 0.95 ± 0.11a | 0.82 ± 0.11a | 0.44 ± 0.14 | 0.34 ± 0.08 | ||
| AHP amplitude (mV) | 16 ± 4a | 17 ± 2a | 26 ± 7 | 25 ± 6 | ||
| Adaptation ratio | 0.42 ± 0.25a | 0.51 ± 0.27a (n = 13) | 0.95 ± 0.22 (n = 31) | 0.94 ± 0.15 | ||
| Input resistance, (MΩ) | 135 ± 39 | 136 ± 55a | 161 ± 49 | 245 ± 78 | ||
| Time constant (ms) | 19 ± 6a | 18 ± 3a | 7 ± 2 | 9 ± 3 | ||
Significantly different from FS interneurons within the same species (P < 0.05).
Membrane properties were very similar in pyramidal cells from rat and monkey PFC (Fig. 1A,B, Table 1), but in both species these properties were significantly different from those of FS interneurons. Pyramidal cell APs had slower time course, larger amplitude and AHPs of smaller amplitude. Unlike FS interneurons, pyramidal cells displayed significant spike frequency adaptation in spike trains evoked by somatic current injection. The membrane time constant was significantly larger and input resistance was significantly lower in pyramidal cells compared to FS interneurons. These electrophysiological characteristics were consistent with the characteristics of pyramidal cells and FS interneurons reported previously in rat (Connors and Gutnick, 1990; Kawaguchi, 1993, 1995) and monkey neocortical slices (Gonzalez-Burgos et al., 2004).
Morphology was recovered for 25 FS interneurons and 15 pyramidal cells in rat PFC slices (Fig. 1C,D,F). The majority of FS interneurons (n = 20) had multipolar aspiny dendrites and axonal arbors that spread in non-preferential directions or predominantly horizontally, features characteristic of the cells with local and horizontal axonal arbors described previously in rat cortex (Kawaguchi, 1995; Fig. 1D). Five FS interneurons were chandelier cells, with characteristic vertical arrangements of axonal boutons and smooth multipolar dendrites (Kawaguchi, 1995; DeFelipe, 2002; Fig. 1F). In monkey PFC, morphology was recovered in 15 FS interneurons and 20 pyramidal cells (Fig. 1E). Morphologically, FS interneurons were represented by local (n = 7), medium (n = 4) (Fig. 1E) and wide arbor (n = 3) neurons, and chandelier cells (n = 1), as previously described in monkey PFC using the Golgi impregnation technique (Lund and Lewis, 1993). The majority of local, medium and wide arbor cells possessed multipolar dendrites. Local arbor cells formed a compact axonal arbor ∼300–500 μm wide. Axonal branches of wide arbor cells spread laterally 900 μm or more. Medium arbor interneurons had an axonal arbor of intermediate width.
Differential Properties of mEPSPs and mEPSCs in Pyramidal Cells and FS Interneurons
To characterize the excitatory inputs to pyramidal cells and FS interneurons, we first compared miniature excitatory responses, which result from spontaneous glutamate release at single synapses.
Intrinsic membrane properties shape the time course and amplitude of EPSPs in different ways in pyramidal cells and interneurons (Stuart and Sakmann, 1995; Fricker and Miles 2000; Galarreta and Hestrin, 2001; Gonzalez-Burgos and Barrionuevo, 2001; Gonzalez-Burgos et al., 2004). Since membrane properties of PFC pyramidal cells and FS interneurons are strikingly different (Fig. 1, Table 1), and therefore could contribute to differential properties of EPSPs in the two cell types, synaptic events were initially recorded in current clamp mode.
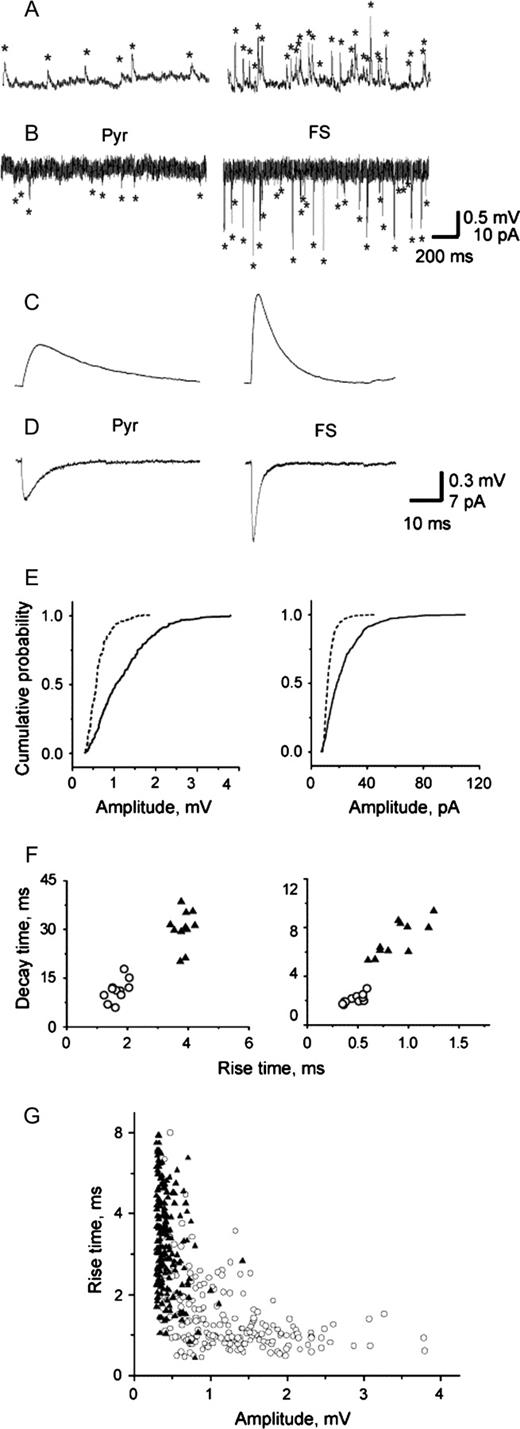
A qualitative assessment of the sweeps suggested significant differences between the mEPSPs recorded from FS interneurons and pyramidal cells (Fig. 2A,C). Quantitative analysis confirmed that in FS interneurons mEPSPs had larger peak amplitude compared to pyramidal cells in both rat and monkey PFC. The cumulative frequency histogram for mEPSP amplitude is less steep for FS interneurons than for pyramidal cells (Fig. 2E), indicating the presence of a greater number of large amplitude events in FS interneurons. The coefficient of variation (CV) estimated for the mEPSP amplitude was larger in FS interneurons than in pyramidal cells (52.8 ± 7.2 versus 36.5 ± 9.3, P < 0.001). FS interneuron mEPSPs exhibited both shorter rise time and decay time constant than pyramidal cells (Fig. 2C, Table 2,). Plotting the mEPSPs decay time against their rise time revealed that FS interneurons and pyramidal cells form two non-overlapping cell populations (Fig. 2F).
Properties of mEPSPs and mEPSCs in pyramidal cells and FS interneurons. Series of mEPSPs (A) and mEPSCs (B) recorded from pyramidal cells (Pyr) and FS interneurons from rat PFC. The average mEPSPs (C) and mEPSCs (D) show that miniature synaptic events had larger amplitude and faster time course in FS interneurons than in pyramidal cells. (E) Cumulative frequency distribution histogram of mEPSPs (left) and mEPSCs (right) amplitude (200 events per cell) in a pyramidal cell (dashed line) and a FS interneuron (solid line) representative of the populations of pyramidal cells and FS interneurons. The amplitude is shifted towards larger values in FS interneurons compared to pyramidal cells for both mEPSPs (K-S Z = 4.6, P < 0.001) and mEPSCs (K-S Z = 7.7, P < 0.001). (F) Plot of mEPSPs (left) and mEPSCs (right) decay time against rise time in FS interneurons (open circles) and pyramidal cells (filled triangles). (G) Graphs of mEPSP amplitude against 10–90% rise time revealed that despite a large degree of overlap in the distributions, a group of fast and large amplitude events was present in FS interneurons (open circles) but absent in pyramidal cells (filled triangles). A weak negative correlation between amplitude and 10–90% rise time was observed in pyramidal cells and FS interneurons (−0.26, P < 0.01 and −0.43, P < 0.01 for these representative examples).
Properties of mEPSPs in pyramidal cells and FS interneurons in the rat and monkey PFC
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 11) . | Monkey (n = 7) . | Rat (n = 11) . | Monkey (n = 8) . | ||
| Amplitude (mV) | 0.52 ± 0.09a | 0.45 ± 0.04a | 0.94 ± 0.21 | 0.93 ± 0.29 | ||
| 10–90% Rise time (ms) | 3.8 ± 0.2a | 3.3 ± 0.2a | 1.7 ± 0.3 | 1.8 ± 0.2 | ||
| Decay time, τ (ms) | 30 ± 6a | 28 ± 5a | 11 ± 3 | 13 ± 5 | ||
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 11) . | Monkey (n = 7) . | Rat (n = 11) . | Monkey (n = 8) . | ||
| Amplitude (mV) | 0.52 ± 0.09a | 0.45 ± 0.04a | 0.94 ± 0.21 | 0.93 ± 0.29 | ||
| 10–90% Rise time (ms) | 3.8 ± 0.2a | 3.3 ± 0.2a | 1.7 ± 0.3 | 1.8 ± 0.2 | ||
| Decay time, τ (ms) | 30 ± 6a | 28 ± 5a | 11 ± 3 | 13 ± 5 | ||
Significantly different from FS interneurons within the same species (P < 0.05).
Properties of mEPSPs in pyramidal cells and FS interneurons in the rat and monkey PFC
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 11) . | Monkey (n = 7) . | Rat (n = 11) . | Monkey (n = 8) . | ||
| Amplitude (mV) | 0.52 ± 0.09a | 0.45 ± 0.04a | 0.94 ± 0.21 | 0.93 ± 0.29 | ||
| 10–90% Rise time (ms) | 3.8 ± 0.2a | 3.3 ± 0.2a | 1.7 ± 0.3 | 1.8 ± 0.2 | ||
| Decay time, τ (ms) | 30 ± 6a | 28 ± 5a | 11 ± 3 | 13 ± 5 | ||
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat (n = 11) . | Monkey (n = 7) . | Rat (n = 11) . | Monkey (n = 8) . | ||
| Amplitude (mV) | 0.52 ± 0.09a | 0.45 ± 0.04a | 0.94 ± 0.21 | 0.93 ± 0.29 | ||
| 10–90% Rise time (ms) | 3.8 ± 0.2a | 3.3 ± 0.2a | 1.7 ± 0.3 | 1.8 ± 0.2 | ||
| Decay time, τ (ms) | 30 ± 6a | 28 ± 5a | 11 ± 3 | 13 ± 5 | ||
Significantly different from FS interneurons within the same species (P < 0.05).
We used voltage clamp recordings in rat PFC slices, first to investigate whether the target-cell specificity of spontaneous synaptic events was still observed under these conditions that increase the ability to detect small amplitude events, and second to address whether the differential properties of mEPSPs in the two cell types may simply be due to their membrane properties. First, we compared the frequencies of mEPSCs and mEPSPs recorded in the same cells. For pyramidal cells, as well as for FS interneurons, the frequency of events recorded under voltage clamp conditions was higher than in current clamp (pyramidal cells: 2.6 ± 1.7 Hz versus 1.8 ± 1.2 Hz, P < 0.03, n = 5; FS interneurons: 25.6 ± 14.7 Hz versus 21.5 ± 14.2 Hz, p < 0.002, n = 4) (Fig. 2B). Second, we found that similar to mEPSPs, mEPSCs in FS interneurons had larger peak amplitudes than those obtained from pyramidal cells (21 ± 4 pA, n = 12 versus 10 ± 2 pA, n = 12; P < 0.001) (Fig. 2D,E). The kinetics of miniature currents were fast in FS interneurons; both rise time (0.45 ± 0.08 ms versus 0.76 ± 0.29 ms, P < 0.001) and decay time constant (2.0 ± 0.2 ms versus 6.5 ± 1.5 ms, P < 0.001) were significantly shorter in FS interneurons than in pyramidal cells. Similarly to mEPSPs, after plotting the mEPSCs decay time against rise time, FS interneurons and pyramidal cells appeared to form two distinct populations (Fig. 2F). Thus, to the extent that voltage-clamp eliminates the effects of membrane properties, the results suggest that membrane properties alone cannot explain the differential properties of mEPSPs in the two cell types.
To assess the differential contribution of cable attenuation to the differences of responses in the two cell types, we analyzed scatter plots of 10–90% rise time versus amplitude (Fig. 2G). Weak negative correlations (−0.2 to −0.43, P < 0.05) were observed in the majority of pyramidal cells and FS interneurons, indicating the presence of dendritic filtering in both groups.
Differential Properties of the Spike-evoked EPSPs and EPSCs in Pyramidal Cells and FS Interneurons
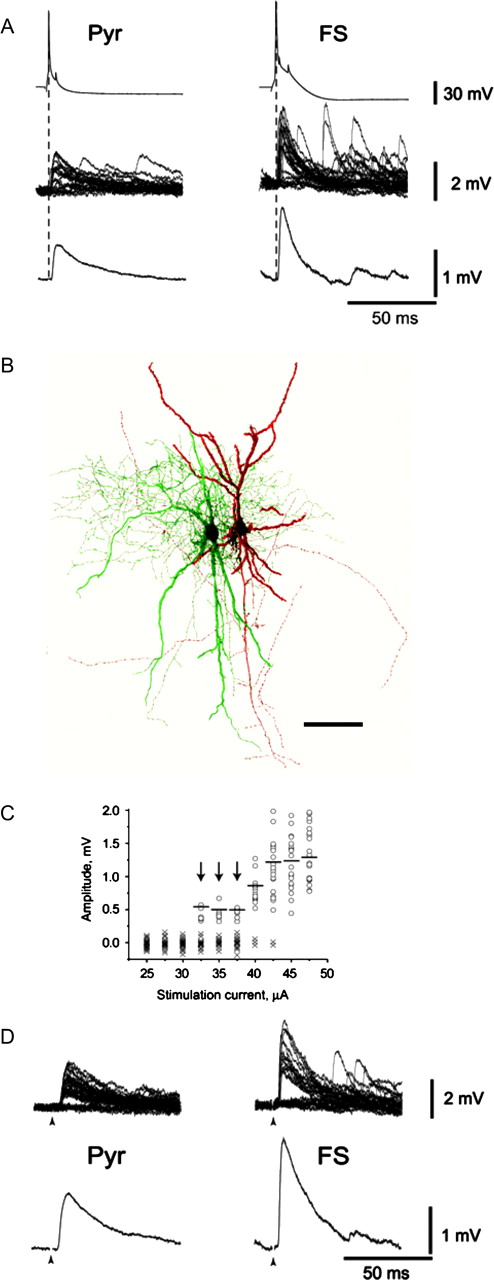
In contrast to mEPSPs, AP-evoked EPSPs are typically associated with simultaneous release from multiple synaptic contacts, since neocortical excitatory synaptic connections are usually multisynaptic (Edwards, 1995; Buhl et al., 1997; Markram et al., 1997; Gil et al., 1999; but see Gulyas et al., 1993; Krimer and Goldman-Rakic, 2001). Hence, somatic EPSPs elicited by stimulation of single axons may result from spatial summation of several single-synapse EPSPs. Since the mechanisms of spatial summation as well as the number of synaptic contacts per connection may differ between pyramidal cells and FS interneurons, the differential properties observed for single-synapse events could be either enhanced or attenuated in multi-synaptic EPSPs. Thus, we examined the amplitude and time course of AP-evoked EPSPs to determine whether they differed between FS interneurons and pyramidal cells in a manner similar to that observed for spontaneous synaptic events. First, we recorded uEPSPs elicited by stimulation of single presynaptic pyramidal cells during simultaneous recordings from synaptically connected pairs. The differential properties observed for uEPSPs recorded from FS interneurons and pyramidal cells were remarkably similar to the differences observed between mEPSPs recorded from rat and monkey PFC neurons (Fig. 3A,B, Table 3). In a second experiment, single-axon responses were elicited in pyramidal cells and FS interneurons by employing extracellular minimal stimulation (Allen and Stevens, 1994; Dobrunz and Stevens, 1997) applied at the border between white matter and layer 6 (Fig. 3C). In cells recorded from rat or monkey PFC, EPSPs evoked with minimal stimulation were larger in peak amplitude and had significantly shorter rise time and decay time constants in FS interneurons compared to pyramidal cells (Fig. 3D; Table 3).
Properties of EPSPs evoked by stimulation of single axons. (A) EPSPs recorded in connected pairs were larger in amplitude and faster in time course in FS interneurons compared to pyramidal cells (Pyr) from rat PFC. (B) Confocal microscope reconstruction of a connected pair of pyramidal cell (red) and FS interneuron (interneuron with long horizontal arbors, green) filled with fluorescent dyes Alexa 568 and 488 during recording in rat PFC (scale bar 100 μm). (C) The amplitude of EPSPs evoked by stimulation from the border of white matter/layer 6 was plotted against stimulation current intensity. EPSPs were considered to result from minimal (single-axon) stimulation when displaying stable amplitude and failure rate, as observed in the 32.5–37.5 μA stimulation current range (arrows) in the representative example of a pyramidal cell in rat PFC (open circles represent responses, crosses represent failures). (D) EPSPs evoked by minimal stimulation had larger amplitude and faster time course in FS interneurons compared to pyramidal cells. Stimulus artifact (arrowhead) is truncated.
Properties of single-axon excitatory responses in pyramidal cells and FS interneurons in the rat and monkey PFC
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat . | Monkey . | Rat . | Monkey . | ||
| uEPSPsa | ||||||
| Amplitude (mV) | 0.55 ± 0.43c | 0.46 ± 0.29c | 1.6 ± 1.6 | 2.2 ± 1.1 | ||
| 10–90% Rise time (ms) | 3.3 ± 1.7c | 3.5 ± 1.9c | 1.9 ± 0.9 | 2.4 ± 1.6 | ||
| Decay time, τ (ms) | 25 ± 7c | 31 ± 9c | 11 ± 4 | 14 ± 9 | ||
| EPSPs evoked by minimal stimulationb | ||||||
| Amplitude (mV) | 0.56 ± 0.22c | 0.60 ± 0.18c | 1.32 ± 0.65 | 1.12 ± 0.04 | ||
| 10–90% Rise time (ms) | 3.1 ± 0.1c | 2.7 ± 0.9c | 1.6 ± 0.4 | 1.6 ± 0.4 | ||
| Decay time, τ (ms) | 20 ± 3c | 28 ± 6c | 12 ± 8 | 11 ± 4 | ||
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat . | Monkey . | Rat . | Monkey . | ||
| uEPSPsa | ||||||
| Amplitude (mV) | 0.55 ± 0.43c | 0.46 ± 0.29c | 1.6 ± 1.6 | 2.2 ± 1.1 | ||
| 10–90% Rise time (ms) | 3.3 ± 1.7c | 3.5 ± 1.9c | 1.9 ± 0.9 | 2.4 ± 1.6 | ||
| Decay time, τ (ms) | 25 ± 7c | 31 ± 9c | 11 ± 4 | 14 ± 9 | ||
| EPSPs evoked by minimal stimulationb | ||||||
| Amplitude (mV) | 0.56 ± 0.22c | 0.60 ± 0.18c | 1.32 ± 0.65 | 1.12 ± 0.04 | ||
| 10–90% Rise time (ms) | 3.1 ± 0.1c | 2.7 ± 0.9c | 1.6 ± 0.4 | 1.6 ± 0.4 | ||
| Decay time, τ (ms) | 20 ± 3c | 28 ± 6c | 12 ± 8 | 11 ± 4 | ||
Number of observations: pyramidal cells: rat, 14; monkey, 9; FS interneurons: rat, 6; monkey, 4.
Number of observations: pyramidal cells: rat, 7; monkey, 6; FS interneurons: rat, 6; monkey, 3.
Significantly different from FS interneurons within the same species (P < 0.05).
Properties of single-axon excitatory responses in pyramidal cells and FS interneurons in the rat and monkey PFC
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat . | Monkey . | Rat . | Monkey . | ||
| uEPSPsa | ||||||
| Amplitude (mV) | 0.55 ± 0.43c | 0.46 ± 0.29c | 1.6 ± 1.6 | 2.2 ± 1.1 | ||
| 10–90% Rise time (ms) | 3.3 ± 1.7c | 3.5 ± 1.9c | 1.9 ± 0.9 | 2.4 ± 1.6 | ||
| Decay time, τ (ms) | 25 ± 7c | 31 ± 9c | 11 ± 4 | 14 ± 9 | ||
| EPSPs evoked by minimal stimulationb | ||||||
| Amplitude (mV) | 0.56 ± 0.22c | 0.60 ± 0.18c | 1.32 ± 0.65 | 1.12 ± 0.04 | ||
| 10–90% Rise time (ms) | 3.1 ± 0.1c | 2.7 ± 0.9c | 1.6 ± 0.4 | 1.6 ± 0.4 | ||
| Decay time, τ (ms) | 20 ± 3c | 28 ± 6c | 12 ± 8 | 11 ± 4 | ||
| . | Pyramidal cells . | . | FS interneurons . | . | ||
|---|---|---|---|---|---|---|
. | Rat . | Monkey . | Rat . | Monkey . | ||
| uEPSPsa | ||||||
| Amplitude (mV) | 0.55 ± 0.43c | 0.46 ± 0.29c | 1.6 ± 1.6 | 2.2 ± 1.1 | ||
| 10–90% Rise time (ms) | 3.3 ± 1.7c | 3.5 ± 1.9c | 1.9 ± 0.9 | 2.4 ± 1.6 | ||
| Decay time, τ (ms) | 25 ± 7c | 31 ± 9c | 11 ± 4 | 14 ± 9 | ||
| EPSPs evoked by minimal stimulationb | ||||||
| Amplitude (mV) | 0.56 ± 0.22c | 0.60 ± 0.18c | 1.32 ± 0.65 | 1.12 ± 0.04 | ||
| 10–90% Rise time (ms) | 3.1 ± 0.1c | 2.7 ± 0.9c | 1.6 ± 0.4 | 1.6 ± 0.4 | ||
| Decay time, τ (ms) | 20 ± 3c | 28 ± 6c | 12 ± 8 | 11 ± 4 | ||
Number of observations: pyramidal cells: rat, 14; monkey, 9; FS interneurons: rat, 6; monkey, 4.
Number of observations: pyramidal cells: rat, 7; monkey, 6; FS interneurons: rat, 6; monkey, 3.
Significantly different from FS interneurons within the same species (P < 0.05).
Because stimulation of a single axon typically elicits subthreshold responses, as shown in vivo (Henze et al., 2002), recruitment of pyramidal cells and FS interneurons requires coincident activation of multiple axons and, hence, summation of the uEPSPs. Because the mechanisms of EPSP summation depend on the EPSP amplitude (Cash and Yuste, 1998, 1999; Urban and Barrionuevo, 1998), compound EPSPs elicited by simultaneous stimulation of more than one presynaptic axon may sum in a different manner compared to small amplitude, single-synapse EPSPs. To determine if the differential properties of EPSPs in FS interneurons versus pyramidal cells were present also in compound EPSPs, we evoked responses that were shown to be glutamate-mediated (Fig. 4A) in the two cell types, by stimulating multiple presynaptic fibers from the border white matter/layer 6.
Compound EPSPs evoked by the stimulation of the border of white matter and layer 6. (A) eEPSPs were isolated by adding BMI (10–20 μM) in the bath solution. Bath application of the glutamate-receptors blockers CNQX (20 μM) and AP5 (50 μM) completely blocked evoked responses in pyramidal cells (Pyr, n = 9) and FS interneurons (n = 7). (B) The amplitude of eEPSPs is plotted against stimulation current intensity for responses recorded from FS interneurons (n = 7, circles) and pyramidal cells (n = 9, triangles). Stimulation current was applied in consecutive steps of 5–10 pA increments. Amplitudes of eEPSPs were averaged for each level of stimulation current. Linear fitting revealed greater proportional increase in eEPSP amplitude in FS interneurons (dashed line) compared to pyramidal cells (solid line). (C) Representative examples of compound eEPSPs (averages of 20 sweeps) recorded from a pyramidal cell–FS interneuron pair in rat PFC. Stimulus artifact (arrowhead) is truncated. (D) Amplitudes of eEPSPs did not differ in the pairs of two pyramidal cells in rat (n = 8, solid line) and monkey (n = 4, dotted line) PFC, but was considerably larger in FS interneurons than in pyramidal cells (E) (n = 14 in rat and n = 4 in monkey). (F) eEPSP in FS interneurons have faster 10–90% rise time (upper graph) and decay time, τ (lower graph), compared to pyramidal cells in rat [n = 15 (Pyr) and 15 (FS)] and monkey [n = 5 (Pyr) and 4 (FS)] PFC. (G) The average eEPSCs as well as the column graphs show that evoked excitatory currents had larger amplitude and faster time course in FS interneurons (n = 5) than in pyramidal cells (n = 6).
In order to compare the amplitude of multi-axonal responses, we utilized two approaches. First, during recordings from single neurons, the stimulus intensity was progressively increased (5–10 μA steps). The amplitude of eEPSPs was plotted against stimulation current intensity normalized relative to stimulus threshold. The relation between eEPSP amplitude and stimulus intensity was fitted well by a linear function in both FS interneurons and pyramidal cells (Fig. 4B). The significantly steeper relation between stimulus intensity and eEPSP amplitude in FS interneurons than in pyramidal cells (the slope was 0.06 mV/μA in pyramidal cells and 0.23 mV/μA in FS interneurons) suggested that the differences between cell types can be also observed for compound EPSPs of increasing amplitude.
Second, responses were evoked simultaneously in pairs of either two pyramidal cells, or of a pyramidal cell and a FS interneuron (Fig. 4C–F). This experimental design enabled us to study simultaneously the behavior of the two cell types in response to extracellular stimulation. Because the minimum stimulus intensity necessary to evoke an eEPSP was comparable in the two cell types across experiments (48 ± 4 μA in FS interneurons, n = 7 and 46 ± 11 μA in pyramidal cells, n = 8), it was possible to perform simultaneous recordings of subthreshold responses in pyramidal cells and FS interneurons. The stimulus intensity employed was low and the response amplitude was small, but typically larger than the amplitude of EPSPs evoked by minimal stimulation, indicating recruitment of more than one presynaptic axon. Contrasting with EPSPs evoked simultaneously in pairs of neighboring pyramidal cells, which had very similar amplitude (Fig. 4D) and time course, eEPSPs had larger amplitudes in FS interneurons than in neighboring pyramidal cells (Fig. 4E). Similar to single-synapse and single-axon EPSPs, compound EPSPs had faster rise and decay time in FS interneurons compared to pyramidal cells (Fig. 4F).
In a similar manner to the miniature events, we addressed the contribution of the membrane properties to the observed differences of the spike-evoked EPSPs in FS interneurons and pyramidal cells. If biophysical properties of the somatic and proximal membrane compartments significantly and differentially shape synaptic events, then under voltage-clamp recording conditions, the differences between cell types should be eliminated or attenuated. In the two cell types we recorded EPSCs evoked by the stimulation of the white matter. As shown in Figure 4G, EPSCs evoked in FS interneurons were significantly faster than in pyramidal cells: they exhibited both shorter rise time and decay time constant.
Suprathreshold Stimulation of FS Interneurons and Disynaptic Inhibitory Response in Pyramidal Cells
The aforementioned properties of EPSPs in FS interneurons — fast kinetics that favor shorter latency EPSP-spike coupling (Fricker and Miles, 2000; Maccaferri and Dingledine, 2002) and large amplitude (that can be expected to let FS interneurons reach spike threshold before pyramidal cells) — enable FS interneurons to provide disynaptic inhibition to pyramidal cells. To model this situation, we applied extracellular synaptic stimulation at the border between white matter and layer 6 and simultaneously recorded pyramidal cells and FS interneurons.
Pyramidal cells that were recorded simultaneously with nearby FS interneurons were depolarized in order to visualize the inhibitory response. Stimulation with relatively high intensities (120–160 μA) typically elicited an EPSP–IPSP sequence in pyramidal cells. The IPSP showed longer and more variable latency than the monosynaptic EPSP and was abolished by the GABAA receptor blocker BMI (10–20 μM; Fig. 5A). Consistent with the disynaptic nature of the IPSP, application of the glutamate receptor antagonists CNQX (20 μM) and AP5 (50 μM) abolished the monosynaptic EPSP and also the IPSP (Fig. 5B). Whereas low stimulus intensities elicited subthreshold EPSPs in both cells, high stimulation currents that evoked a disynaptic IPSP (dIPSP) in the pyramidal cells simultaneously evoked an AP from the resting membrane potential in the nearby FS interneuron in seven out of nine recorded pairs in rat and in the one pair recorded in monkey (Fig. 5C).
Properties of disynaptic inhibitory responses in pyramidal cells and suprathreshold stimulation of FS interneurons. (A) Relatively high stimulus intensities evoked in pyramidal cells an EPSP–IPSP sequence. The dIPSP (arrow) was abolished by application of the GABAA receptor antagonist BMI. [Stimulus artifact (arrowhead) is truncated.] (B) The disynaptic nature of the IPSP was indicated by its elimination after application of the glutamate receptor antagonists CNQX and AP5. (C) Low-intensity stimulation evoked subthreshold EPSPs in both cells of a simultaneously recorded pair of FS cell and pyramidal neuron (note that the decay time of the pyramidal cell EPSP is strongly voltage-sensitive and that the EPSP in the pyramidal cell is much slower when compared to the values obtained at resting membrane potential; cf. Table 3). An increase in stimulation current evoked an EPSP–IPSP sequence in the pyramidal cell and simultaneous spiking in the FS interneuron. Note that the spike in the FS cell precedes the onset of the IPSP in the pyramidal neuron. (D) The latency of dIPSP measured from the stimulus artifact to the onset of dIPSP, decreased when pyramidal cells were depolarized close to the level of monosynaptic EPSP reversal potential (arrows show the dIPSP onset). Note that the spike peak in FS interneuron still precedes dIPSP onset. (E) In the representative example from rat PFC uIPSP and dIPSP in the pyramidal cell were recorded in consecutive experiments. APs in the FS interneuron are superimposed. (F) The latency of the uIPSP obtained from connected pairs of FS interneurons–pyramidal cells from rat and monkey (empty column, n = 8) was similar to the latency of the dIPSPs (hatched column, n = 7).
The spike in the FS interneuron appeared prior to the dIPSP (Fig. 5D). However, at membrane potentials close to −50 mV the latency of the dIPSP could be overestimated because the preceding monosynaptic EPSP might obscure the onset of the dIPSP. Therefore, in a number of pyramidal cells (n = 7) we added QX-314 (5 mM) to the intracellular solution to block voltage-gated sodium channels and depolarized them further near the reversal potential for glutamate-mediated responses. At this membrane potential, the EPSP amplitude was close to 0 mV and the onset of the dIPSP could be clearly identified. Although in this condition the onset of dIPSP was detected earlier than at potentials close to −50 mV, it still followed the peak of AP in FS interneurons (Fig. 5D).
To test if the time from the peak of AP in FS interneurons to the onset of dIPSP in pyramidal cells was compatible with the monosynaptic latency of uIPSPs elicited by FS interneurons in pyramidal cells, we recorded inhibitory responses in connected pairs of FS interneurons and pyramidal cells from rat and monkey PFC. uIPSP data from monkey (n = 4) and rat (n = 4) were pooled as no differences in their properties were observed. The latency was 1.02 ± 0.14 ms for uIPSPs in monkey slices compared to 0.92 ± 0.18 ms in inhibitory connections recorded in the rat. Similarly, neither amplitude (monkey: 0.61 ± 0.31 mV; rat: 0.64 ± 0.32 mV) nor 10–90% rise time (monkey: 2.2 ± 0.9 ms; rat: 2.3 ± 1.1 ms) differed significantly. The latency of the dIPSPs (measured at potentials close to EPSP reversal) appeared to be similar to the latency of the unitary IPSPs (Fig. 5F).
Discussion
We found that excitatory synaptic responses in FS interneurons and pyramidal cells in the PFC possessed differential, target-cell-specific properties. Miniature excitatory responses, presumably representing the response to glutamate release at single synapses, were larger and faster in FS interneurons than in pyramidal cells. Similar differences were observed for spike-evoked EPSPs elicited by the stimulation of single axons in connected pairs or with minimal stimulation. Recruitment of multiple fibers by extracellular stimulation up to the firing threshold of FS interneurons demonstrated that compound EPSPs elicited by stimulation of multiple axons possessed differential properties similar to those of single-synapse and single-axon responses. These differential properties of the postsynaptic excitatory responses enabled FS interneurons to generate APs earlier than pyramidal cells, and thus to elicit dIPSPs in pyramidal cells, in a manner consistent with the mechanisms of feed-forward inhibition thought to operate in neural circuits of the hippocampus and primary sensory cortices (Buzsaki, 1984; Hirsch and Gilbert, 1991; Porter et al., 2001; Pouille and Scanziani, 2001; Swadlow, 2003).
Similar results were found in both rat and monkey PFC, indicating a stable role of FS interneurons in neocortical functioning across species, despite the marked differentiation of the primate PFC and the rodent–primate differences in the relative percentage of GABA neurons with FS characteristics (Kawaguchi, 1995; Gabbott and Bacon, 1996; Fuster, 1997; Gabbott et al., 1997; Kawaguchi and Kubota; 1997; Zaitsev et al., 2005). In addition, the differential recruitment of FS interneurons and pyramidal cells by incoming excitation was similar in monkey and rat PFC. These results are consistent with the idea that the differences in membrane properties between these two cell types are conserved across a wide range of species, including rodents (McCormick et al., 1985; Connors and Gutnick, 1990; Kawaguchi, 1995), monkeys (Gonzalez-Burgos et al., 2004; Zaitsev et al., 2005) and humans (Avoli and Olivier, 1989; Foehring et al., 1991; Tasker et al., 1996; Menendez de la Prida et al., 2002).
Differential Properties of Excitatory Subthreshold Responses in FS Interneurons and Pyramidal Cells
All subthreshold excitatory responses recorded in the rat and monkey PFC — mEPSPs, single-axon uEPSPs and eEPSPs — had larger amplitude and faster time course in FS interneurons compared to pyramidal cells. Our data are in accordance with a number of previous studies in other cortical areas. For example, in layers 2–3 and 4 of rat somatosensory and visual cortex, local excitatory responses were larger and faster in FS interneurons compared to pyramidal cells (Beierlein et al., 2003; Holmgren et al., 2003). This also appeared to hold true for unitary connections in layers 2–3 from medial PFC of ferrets (Gao et al., 2003). Similarly, minimal stimulation of thalamic inputs to layers 4 and 5 of somatosensory cortex evoked larger and faster subthreshold excitatory responses in FS interneurons than in pyramidal cells (Gibson et al., 1999; Beierlein et al., 2003). However, uEPSPs in layers 2–5 from rat neocortex had comparable amplitudes in FS interneurons and pyramidal cells, although the responses were still faster in FS interneurons (Thomson, 1997).
Several factors can potentially contribute to the observed differences between FS interneurons and pyramidal cells. For example, as shown here and by others (Connors and Gutnick, 1990; Kawaguchi, 1993; Gonzalez-Burgos et al., 2004), membrane properties of pyramidal cells and FS interneurons differ remarkably from each other. Since these properties are important in shaping amplitude and kinetic of subthreshold responses (Rall, 1967; Stuart and Sakmann, 1995; Geiger et al., 1997; Markram et al., 1997; Galarreta and Hestrin, 2001; Gonzalez-Burgos and Barrionuevo, 2001; Gonzalez-Burgos et al., 2004), they may contribute to the differential properties of the EPSPs in pyramidal cells and FS interneurons. For example, a shorter time constant in FS interneurons could yield faster EPSP kinetics, whereas a larger input resistance in FS interneurons could boost the EPSP amplitude.
It is unlikely, however, that biophysical properties of the perisomatic membrane are the only factor responsible for the observed differences in the EPSPs. Indeed, similar results were obtained in our study for miniature and evoked compound excitatory postsynaptic currents, which presumably are less affected by the biophysical properties of the somatic membrane due to recording in somatic voltage-clamp conditions. The potential synaptic mechanisms underlying the observed properties of the EPSCs may involve presynaptic machinery [e.g. target-cell specific release probability (Koester and Johnston, 2005)] as well as postsynaptic mechanisms. Thus, the AMPA-receptors in FS interneurons have a distinct subunit composition and, hence, biophysical properties which can decrease the time course of channel deactivation and increase single channel conductance (Hestrin, 1993; Jonas et al., 1994; Geiger et al., 1995, 1997; Koh et al., 1995; Angulo et al., 1999). Hence, properties of synaptic currents can partially contribute to the observed differences of EPSPs properties.
Since similar differential properties of EPSCs have been observed for miniature responses and responses evoked by white matter stimulation, which might differ in their origin (Sara et al., 2005), it is likely that our observations apply to different sources of excitatory inputs. In addition, it was previously shown that unitary EPSCs, which represent exclusively local excitatory connections, appear to be faster in FS interneurons, thus indicating that synaptic properties contribute to the cell-type specific differences in responses for this kind of input as well (Geiger et al., 1997, Angulo et al., 1999; Aaron and Dichter, 2001).
Another factor to consider is the location of synaptic contacts. Numerous studies indicate that in interneurons, excitatory synapses are located not only on dendrites, but also on soma, whereas in pyramidal cells, the cell body does not receive excitatory synapses (Schwartzkroin and Kunkel, 1985; Seress and Ribak, 1985; Ahmed et al., 1997; Somogyi et al., 1998). Furthermore, the dendritic tree of interneurons may be considerably more compact than that of pyramidal cells. Thus, in FS interneurons many excitatory responses could be generated by synapses that are significantly more proximal than in pyramidal cells and therefore would be less filtered by the dendritic cable properties. Indeed, we observed in FS interneurons (but not in pyramidal cells) a large fraction of mEPSPs with significantly larger amplitude and faster rise time (Fig. 2G), consistent with a small degree of dendritic filtering.
According to our data, an equal increase in stimulation current resulted in a greater increase in evoked EPSP amplitude in FS interneurons than in pyramidal cells. These differences can be explained in part by the observed differences in the amplitude of single-synapse responses. But, in addition the amplitude of compound excitatory responses can be influenced by the number of fibers that form synapses with FS interneurons and pyramidal cells. For example, in rat neocortex the number of connections made by pyramidal cells onto FS interneurons is considerably higher than onto other pyramidal cells (Holmgren et al., 2003). However, for our data we cannot estimate precisely the number of fibers involved in the generation of excitatory responses in the two cell types.
Differential Properties of EPSPs in FS Interneurons and Pyramidal Cells Could Provide Basis for the Disynaptic Inhibition of Pyramidal Cells
The observed properties of subthreshold excitatory responses, such as large amplitude and fast kinetics, could reflect a specific role of FS interneurons in PFC circuitry. For example, fast kinetics (both rise and decay time) of EPSPs are important factors that contribute to the short latency of EPSP-spike coupling in neurons (Fricker and Miles, 2000; Maccaferri and Dingledine, 2002), which in turn provides temporal precision of AP generation (Galarreta and Hestrin, 2001). Therefore, FS interneurons that receive fast and large EPSPs with high reliability should fire with shorter latency than pyramidal cells and would provide the latter with disynaptic inhibitory control.
In both monkey and rat PFC we observed disynaptic inhibitory responses that followed excitatory responses in pyramidal cells. Simultaneous recordings from neighboring FS interneuron–pyramidal cell pairs showed that APs in FS interneurons preceded the onset of dIPSP in pyramidal cells. The latencies of dIPSPs were comparable with the latencies of uIPSPs elicited by FS interneurons in pyramidal cells. These data are consistent with the involvement of FS interneurons in feed-forward inhibition of pyramidal cells.
A unique role of FS interneurons in feed-forward disynaptic inhibition is supported by data from other cortical areas. In rat hippocampus, ‘fast’ interneurons that could restrict spike generation in pyramidal cells when activated by incoming afferents fired earlier than pyramidal cells. In contrast, ‘slow’ interneurons fired at the same time or later than pyramidal cells (Maccaferri and Dingledine, 2002; Lawrence and McBain, 2003). In rat somatosensory cortex, dIPSPs evoked in pyramidal cells by thalamic stimulation showed the same short-term dynamics as thalamically evoked EPSPs in FS interneurons (Beierlein et al., 2003). In rabbit barrel cortex divergent inputs from the thalamus to FS interneurons enabled a sharply synchronous activity that generated only a brief window of opportunity for the spike generation in their post-synaptic targets (Swadlow, 2003).
Implications for the Working Memory Mechanisms and the Pathophysiology of Schizophrenia
Inhibitory neurons appear to be a critical element of the dorsolateral PFC circuitry underlying working memory (Goldman-Rakic, 1995, 1996; Rao et al., 1999, 2000; Wang et al., 2004). Indeed, injection of bicuculline into monkey PFC disturbs performance on working memory tasks (Sawaguchi et al., 1988, 1989) and the tuning of neurons during these tasks (Rao et al., 2000). GABAergic currents seem to be necessary to regulate the persistent activity of principal cells during the delay period of working memory (Durstewitz et al., 2000; Seamans et al., 2003). Furthermore, inhibition can shape the temporal flow of information in the PFC and is detected during all epochs of working memory tasks (Constantinidis et al., 2002). The critical role of FS interneurons in sharpening the tuning of pyramidal cells during spatial working memory tasks has been suggested by modeling studies (Tanaka, 1999; Wang et al., 2004), and also in experiments with GABAA blockade that resulted in expansion of receptive fields in prefrontal cortical neurons (Rao et al., 2000).
In visual and somatosensory cortices FS interneurons serve to sharpen orientation selectivity (Miller et al., 2001; Roerig et al., 2003; Shapley et al., 2003). In a similar fashion, in the monkey PFC, FS interneurons could contribute to the time control of pyramidal cells through more effective involvement by excitation. That is, in the monkey PFC the activity of FS interneurons might sharpen spatial ‘cognitive’ tuning in pyramidal cells, similar to the orientation tuning of pyramidal cells in sensory cortices. In the monkey dorsolateral PFC, FS neurons selectively express the calcium-binding protein parvalbumin (Zaitsev et al., 2005). Consequently, pathological abnormalities that affect parvalbumin-containing interneurons in subjects with schizophrenia (Hashimoto et al., 2003) might result in the breakdown of such pyramidal cell tuning and might thus underlie some of the cognitive deficits present in schizophrenia (Lewis et al., 2005).
The authors thank Dr Takanori Hashimoto for useful comments on the manuscript, Dr Simon Watkins, Mr Stuart Shand and Mr Sean Alber for assistance with confocal reconstructions, and Mrs Olga Krimer and Mr James Kosakowski for their excellent technical assistance. The project was supported by Grants MH067963 and MH51234 from the National Institutes of Health.
References
Aaron GB, Dichter MA (
Ahmed B, Anderson JC, Martin KA, Nelson JC (
Allen C, Stevens CF (
Angulo MC, Rossier J, Audinat E (
Avoli M, Olivier A (
Beierlein M, Gibson JR, Connors BW (
Buhl EH, Tamas G, Szilagyi T, Stricker C, Paulsen O, Somogyi P (
Cash S, Yuste R (
Cash S, Yuste R (
Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA (
Connors BW, Gutnick MJ (
Constantinidis C, Goldman-Rakic PS (
Constantinidis C, Williams GV, Goldman-Rakic PS (
Dobrunz LE, Stevens CF (
Durstewitz D, Seamans JK, Sejnowski TJ (
Edwards FA (
Foehring RC, Lorenzon NM, Herron P, Wilson CJ (
Fricker D, Miles R (
Funahashi S, Bruce CJ, Goldman-Rakic PS (
Fuster JM (
Gabbott PL, Bacon SJ (
Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ (
Galarreta M, Hestrin S (
Gao WJ, Wang Y, Goldman-Rakic PS (
Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H (
Geiger JR, Lubke J, Roth A, Frotscher M, Jonas P (
Gibson JR, Beierlein M, Connors BW (
Gil Z, Connors BW, Amitai Y (
Goldman-Rakic PS (
Gonzalez-Burgos G, Barrionuevo G (
Gonzalez-Burgos G, Barrionuevo G, Lewis DA (
Gonzalez-Burgos G, Krimer LS, Urban NN, Barrionuevo G, Lewis DA (
Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA (
Gulyas AI, Miles R, Sik A, Toth K, Tamamaki N, Freund TF (
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA (
Henze DA, Wittner L, Buzsaki G (
Hestrin S (
Hirsch JA, Gilbert CD (
Holmgren C, Harkany T, Svennenfors B, Zilberter Y (
Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H (
Kawaguchi Y (
Kawaguchi Y (
Kawaguchi Y, Kubota Y (
Koester HJ, Johnston D (
Koh DS, Geiger JR, Jonas P, Sakmann B (
Krimer LS, Goldman-Rakic PS (
Lawrence JJ, McBain CJ (
Letinic K, Zoncu R, Rakic P (
Lewis DA, Hashimoto T, Volk DW (
Lund JS, Lewis DA (
Maccaferri G, Dingledine R (
Markram H, Lubke J, Frotscher M, Roth A, Sakmann B (
McCormick DA, Connors BW, Lighthall JW, Prince DA (
Menendez de la Prida L, Benavides-Piccione R, Sola R, Pozo MA (
Miller EK, Cohen JD (
Miller KD, Pinto DJ, Simons DJ (
Paxinos G, Watson C (
Porter JT, Johnson CK, Agmon A (
Pouille F, Scanziani M (
Rall W (
Rao SG, Williams GV, Goldman-Rakic PS (
Rao SG, Williams GV, Goldman-Rakic PS (
Roerig B, Chen B, Kao JP (
Sara Y, Virmani T, Deak F, Liu X, Kavalali ET (
Sawaguchi T, Matsumura M, Kubota K (
Sawaguchi T, Matsumura M, Kubota K (
Schwartzkroin PA, Kunkel DD (
Seamans JK, Nogueira L, Lavin A (
Seress L, Ribak CE (
Shapley R, Hawken M, Ringach DL (
Simons DJ (
Simons DJ, Carvell GE (
Somogyi P, Tamas G, Lujan R, Buhl EH (
Stuart G, Sakmann B (
Swadlow HA (
Swadlow HA (
Tanaka S (
Tasker JG, Hoffman NW, Kim YI, Fisher RS, Peacock WJ, Dudek FE (
Thomson AM (
Urban NN, Barrionuevo G (
Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS (
Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS (
Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA (
Author notes
1Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA 15213, USA and 2Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15213, USA


![Compound EPSPs evoked by the stimulation of the border of white matter and layer 6. (A) eEPSPs were isolated by adding BMI (10–20 μM) in the bath solution. Bath application of the glutamate-receptors blockers CNQX (20 μM) and AP5 (50 μM) completely blocked evoked responses in pyramidal cells (Pyr, n = 9) and FS interneurons (n = 7). (B) The amplitude of eEPSPs is plotted against stimulation current intensity for responses recorded from FS interneurons (n = 7, circles) and pyramidal cells (n = 9, triangles). Stimulation current was applied in consecutive steps of 5–10 pA increments. Amplitudes of eEPSPs were averaged for each level of stimulation current. Linear fitting revealed greater proportional increase in eEPSP amplitude in FS interneurons (dashed line) compared to pyramidal cells (solid line). (C) Representative examples of compound eEPSPs (averages of 20 sweeps) recorded from a pyramidal cell–FS interneuron pair in rat PFC. Stimulus artifact (arrowhead) is truncated. (D) Amplitudes of eEPSPs did not differ in the pairs of two pyramidal cells in rat (n = 8, solid line) and monkey (n = 4, dotted line) PFC, but was considerably larger in FS interneurons than in pyramidal cells (E) (n = 14 in rat and n = 4 in monkey). (F) eEPSP in FS interneurons have faster 10–90% rise time (upper graph) and decay time, τ (lower graph), compared to pyramidal cells in rat [n = 15 (Pyr) and 15 (FS)] and monkey [n = 5 (Pyr) and 4 (FS)] PFC. (G) The average eEPSCs as well as the column graphs show that evoked excitatory currents had larger amplitude and faster time course in FS interneurons (n = 5) than in pyramidal cells (n = 6).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cercor/16/4/10.1093/cercor/bhj002/2/m_cercorbhj002f04_lw.jpeg?Expires=1716475773&Signature=KD~Kf-GWRYbS3Ew5MTzdtxJEocpZNeAYz0BHr-Snyqr~RJ5ey-KJflbfo0vKpl9NZ-LI1RgaenR3G3veKSjPTqUXmSDJUarZ~HkIzLSGDbTr9nGjsy283rUB3J4OfbaToLMqTOJIlYjxmmWMle1CYEROWw4qv5AzCNCtkM9YYnyuqAy9~Wr1gBDiwFOo67TvsJ2wnY3ZHi9TCXaLWWVMUSPQZyzxZd2V6cv~p8Nl-NamY77SxIWTWSg-pexG5LX7AbgeXM8vHfvJkF8PoC8UkrO2TWOIZmDbhtgLbOl1hpSUPIZiK5ht4o89WtvF1-1ec33vOjkAKRDHJcovYpJ0WQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Properties of disynaptic inhibitory responses in pyramidal cells and suprathreshold stimulation of FS interneurons. (A) Relatively high stimulus intensities evoked in pyramidal cells an EPSP–IPSP sequence. The dIPSP (arrow) was abolished by application of the GABAA receptor antagonist BMI. [Stimulus artifact (arrowhead) is truncated.] (B) The disynaptic nature of the IPSP was indicated by its elimination after application of the glutamate receptor antagonists CNQX and AP5. (C) Low-intensity stimulation evoked subthreshold EPSPs in both cells of a simultaneously recorded pair of FS cell and pyramidal neuron (note that the decay time of the pyramidal cell EPSP is strongly voltage-sensitive and that the EPSP in the pyramidal cell is much slower when compared to the values obtained at resting membrane potential; cf. Table 3). An increase in stimulation current evoked an EPSP–IPSP sequence in the pyramidal cell and simultaneous spiking in the FS interneuron. Note that the spike in the FS cell precedes the onset of the IPSP in the pyramidal neuron. (D) The latency of dIPSP measured from the stimulus artifact to the onset of dIPSP, decreased when pyramidal cells were depolarized close to the level of monosynaptic EPSP reversal potential (arrows show the dIPSP onset). Note that the spike peak in FS interneuron still precedes dIPSP onset. (E) In the representative example from rat PFC uIPSP and dIPSP in the pyramidal cell were recorded in consecutive experiments. APs in the FS interneuron are superimposed. (F) The latency of the uIPSP obtained from connected pairs of FS interneurons–pyramidal cells from rat and monkey (empty column, n = 8) was similar to the latency of the dIPSPs (hatched column, n = 7).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cercor/16/4/10.1093/cercor/bhj002/2/m_cercorbhj002f05_lw.jpeg?Expires=1716475773&Signature=AxFt6N7J5Bfisc9K89rQhdDA8FsI24FlcBnhla9WkVpU5cl986u-WYvZyB3B4gCGShQtkXyp20QfpE86hvaxtUaMUA8-di8SZGbMmRKV2PeKmwHK97TWwqlNB~nWfoKtqZ1VSXMgOYRjHHBRSlU46aihKS5skbB0Zv7lX6gX3r3VwDMpvcURqGR9oxzRJdGOPTzlzw0ej0UN0ikj3KbvTe9az7GnjbXIr5R38M4jXCgM2BO0mLXf55i1KKSNvRxW9723DGjmD0n60sY2Zw9eb~iReyFch5m6yYSgcy8HD~JdBGN7A93oWDiFB3gvU1txBhDfirOtwPJ0EWMXRvP0kg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)