-
PDF
- Split View
-
Views
-
Cite
Cite
Christine Schiltz, Bettina Sorger, Roberto Caldara, Fatima Ahmed, Eugene Mayer, Rainer Goebel, Bruno Rossion, Impaired Face Discrimination in Acquired Prosopagnosia Is Associated with Abnormal Response to Individual Faces in the Right Middle Fusiform Gyrus, Cerebral Cortex, Volume 16, Issue 4, April 2006, Pages 574–586, https://doi.org/10.1093/cercor/bhj005
Close - Share Icon Share
Abstract
The middle fusiform gyrus (MFG) and the inferior occipital gyrus (IOG) are activated by both detection and identification of faces. Paradoxically, patients with acquired prosopagnosia following lesions to either of these regions in the right hemisphere cannot identify faces, but can still detect faces. Here we acquired functional magnetic resonance imaging (fMRI) data during face processing in a patient presenting a specific deficit in individual face recognition, following lesions encompassing the right IOG. Using an adaptation paradigm we show that the fMRI signal in the rMFG of the patient, while being larger in response to faces as compared to objects, does not differ between conditions presenting identical and distinct faces, in contrast to the larger response to distinct faces observed in controls. These results suggest that individual discrimination of faces critically depends on the integrity of both the rMFG and the rIOG, which may interact through re-entrant cortical connections in the normal brain.
Introduction
Humans are exceedingly efficient in discriminating faces, both at the category level (‘It's a face’) and at the individual level (‘It's Peter’) (Tanaka, 2001; Grill-Spector et al., 2004). In attempting to clarify the neuronal mechanisms underlying these complex discrimination abilities, neuroimaging studies have shown that the middle fusiform and inferior occipital gyri consistently yield significant activations when healthy adults view faces compared to other objects, with a right hemispheric dominance (e.g. Sergent et al., 1992; Kanwisher et al., 1997; Halgren et al., 1999; Gauthier et al., 2000; Rossion et al., 2000; see Haxby et al., 2000 for a review). Recent evidence suggests that these two regions of the ventral visual pathway, besides being involved in detecting the presence of a face, also play a role in discriminating individual faces (Gauthier et al., 2000; Eger et al., 2004; Grill-Spector et al., 2004; Rotshtein et al., 2005). However, the precise function(s) of these regions and the nature of their interaction with respect to face detection and discrimination remain(s) largely unresolved.
Given that the middle fusiform gyrus (rMFG) and the inferior occipital gyrus (rIOG) of the right hemisphere are activated by both face detection and individuation it is puzzling that brain damage can impair face identification while leaving face detection intact, as in most cases of prosopagnosia (e.g. Damasio et al., 1982; Gauthier et al., 1999). [These two functional regions, defined by a comparison of faces and non-face stimuli, are also referred to in the literature as the ‘fusiform face area’, or ‘FFA’ (Kanwisher et al., 1997) and the ‘occipital face area, or OFA’ (e.g. Gauthier et al., 2000). Even though this terminology is widely used, it is also somewhat misleading, as these regions do respond to other stimuli than faces and to a different level to distinct objects (e.g. Ishai et al., 2000; Grill-Spector et al., 2004).] Prosopagnosia is classically defined as the inability to recognize faces of conspecifics despite normal intellectual abilities and apparently normal recognition of other object categories (Bodamer, 1947; Farah, 1990; Sergent and Signoret, 1992; Gauthier et al., 1999; Clarke et al., 1997; Laeng and Caviness, 2001). The lesions causing acquired prosopagnosia can be limited to the right hemisphere (Landis et al., 1988; Sergent and Signoret, 1992; Uttner et al., 2002) and are usually found in ventral occipito-temporal cortex, involving both or either of the inferior occipital and fusiform gyri (Damasio et al., 1982; Sergent and Signoret, 1992; Barton et al., 2002).
Defining the critical roles of the rMFG and the rIOG during face processing would provide a substantial contribution towards resolving the apparent paradox between, on the one hand, neuroimaging data showing that these two visual areas are activated by both face detection and individuation and, on the other hand, neuropsychological reports of patients impaired in individual face discrimination but still able to process faces at the categorical level after brain damage.
To investigate the critical role(s) of the rMFG and the rIOG in face detection and individual recognition we acquired functional magnetic resonance imaging (fMRI) data in a single-case brain-damaged prosopagnosic patient, P.S., presenting a deficit restricted to the individual discrimination and recognition of faces (Rossion et al., 2003; Caldara et al., 2005). Importantly, the patient's face detection capacity is intact. Strikingly, her ability to perform within-category identification of stimuli from any object class other than faces is also in the normal range, even though she may show response biases in ‘same/different’ tasks and be slightly slowed down in visual discrimination tasks on nonface stimuli compared to normal controls (Rossion et al., 2003). Most importantly, at the anatomical level, her right hemisphere lesion encompasses the inferior occipital cortex but spares the mid-fusiform gyrus. This is of particular interest because the lesions underlying prosopagnosia are often more widely spread (e.g. Sergent and Signoret, 1992) and concern the right middle fusiform in a large number of cases (e.g. Barton et al., 2002). Moreover, these patients generally present associated deficits in object recognition (Damasio et al., 1982; Gauthier et al., 1999; Laeng and Caviness, 2001). Thus, measuring neural activation in P.S.'s intact brain areas is an exceptional means to study the functional neuro-anatomy of the observed dissociation between intact face detection and impaired individual discrimination restricted to faces.
To test the hypothesis that face-sensitive neurons in the rMFG of P.S. can no longer process facial identity while they are still subserving face detection, we conducted two experiments using fMR adaptation (Grill-Spector et al., 1999; Kourtzi and Kanwisher, 2000; Grill-Spector and Malach, 2001; Henson, 2003) in P.S. and in a group of normal controls. Following the rationale of the adaptation paradigm, specifically the regions coding facial identity yield a larger blood oxygenation level-dependent (BOLD) signal in response to blocks or pairs of trials displaying different individual faces as compared to blocks or pairs of trials with identical faces (Gauthier et al., 2000; Henson et al., 2000; Grill-Spector and Malach, 2001; Eger et al., 2004; Winston et al., 2004; Rotshtein et al., 2005). The recovery from fMR adaptation to facial identity observed in a face-sensitive cortical area is taken as evidence that different facial identities are represented in this region by distinct neuronal response patterns (see Grill-Spector and Malach, 2001; Henson, 2003).
Here we observed a normal response to faces at the categorical level in P.S.'s right mid-fusiform gyrus but a failure of recovery from fMR adaptation to facial identity in the same region. This dissociation between two functions, namely intact face detection and impaired face discrimination in the same cortical area, the rMFG, is in line with the behavior of the prosopagnosic patient P.S. and suggests a lack of contrast between population responses for different face identities in this region. More generally, these findings suggest that the discrimination of individual faces depends critically on the integrity of the two ventral visual regions, which may interact functionally through re-entrant cortical connections.
Materials and Methods
Subjects
The prosopagnosic patient P.S. has been described in detail in Rossion et al. (2003; see also Caldara et al., 2005) and will only be briefly described here. P.S. was born in 1950 and sustained a closed head injury in 1992 which left her with extensive lesions of the left mid-ventral (mainly fusiform gyrus) and the right inferior occipital cortex (Fig. 3A). Minor damages to the left posterior cerebellum and the right middle temporal gyrus were also detected on high resolution T1-weighted anatomical images of her brain. After medical treatment and neuropsychological rehabilitation, P.S. recovered extremely well from her cognitive deficits following the accident (Mayer et al., 1999). Her only continuing complaint remains a profound difficulty in recognizing faces, including those of her family, as well as her own face. To determine a person's identity, she relies on external (non-face-inherent) cues such as haircut, moustache or glasses, but also on the person's voice, posture, gait, etc. The Benton Face Recognition Test (BFRT) (Benton and Van Allen, 1972) ranks her as highly impaired, and in addition her score at the Warrington Recognition Memory Test (WRMT) (Warrington, 1984) for faces characterizes her as significantly less accurate than controls (see Table 1 in Rossion et al., 2003). P.S. does not present any difficulty in recognizing objects, even at the subordinate level (Rossion et al., 2003). However, she states that she reads slower than she did prior to the accident, and she mentions certain difficulties in visual orthography. P.S.'s visual field is almost full (small right paracentral scotoma) and her visual acuity is good (0.8 for both eyes as tested in August 2003), but she is slightly slower than normal subjects at detecting letters and numbers in her right visual field. She is also slower than normals at a simple reaction time task.
Besides P.S., a group of seven age-matched females (age range 49–56 years) performed the behavioral experiment. A total of 13 control subjects participated in the two imaging experiments. Twelve subjects served as controls in experiment 1, and six of these subjects also took part in experiment 2. For experiment 1, we scanned three age-matched female controls, two of whom also participated in experiment 2. However, we had to discard the data of one age-matched subject in experiment 2 because of excessive head movements. [Note that, whereas it was clearly important to test all age-matched controls in a behavioural task measuring RTs, this was not a requisite for the neuroimaging experiments, especially since the profile of activation in the right middle fusiform gyrus remains stable across decades (Brodtmann et al., 2003). As a matter of fact, the profile of response for age-matched controls in the present fMRI experiments did not appear to differ from young controls.] P.S. and the control subjects gave their informed written consent prior to the fMRI experiments. The study was conformed to the Declaration of Helsinki and was approved by the Ethics Committee of the Medical Department of the University of Louvain. All subjects proved to be strongly right-handed according to the Edinburgh Inventory (Oldfield, 1971).
Stimuli and Procedures of the Behavioral Experiment
Five categories of stimuli were used: pictures of faces, cars, chairs, boats and birds (Fig. 1). Twenty-four individual items were used for each category. All images were presented in grayscale, and sustained a size of roughly 4° (faces, chairs) or 4.5° (boats, cars, birds) of visual angle. Faces (half male) were cropped so that no external features (hair, etc.) were present. The subjects were presented with a two-alternative forced-choice (2AFC) matching task. A first stimulus was presented in the centre of the screen for 1000 ms, followed after 1000 ms of blank screen by a pair of stimuli remaining on the screen until the subject's response. One of the items of the pair was the same as the first one, and the other one was a distractor. The distractor could be either from another category (four possibilities, six trials of each) or from the same category (e.g. two faces). Thus, there were 10 conditions: two levels of discrimination (‘categorical discrimination’ and ‘individual discrimination’) × five categories; and 24 trials by condition. Participants were required to identify the target item in the pair as correctly and as fast as possible by pressing the left or right of two keys. The pair of stimuli remained on the screen until the subject's response. Trials were spaced by 1000 ms. A few practice trials were presented before the beginning of the experiment. The left and right positions of the target stimuli were counterbalanced across test items and participants received no feedback for their responses. The whole experiment was divided in two blocks of 120 trials and lasted for ∼25 min.
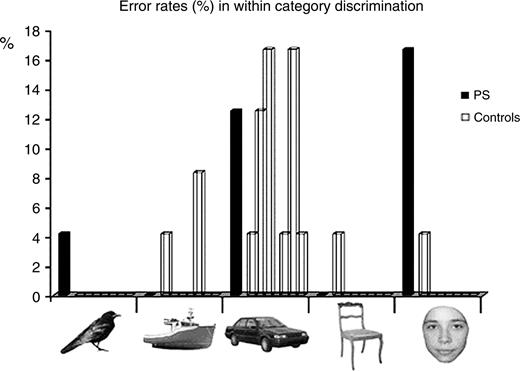
Performance (error rates) in a within-category object-matching task. The black columns represent the error percentage of P.S. and the transparent columns depict performance of the seven age-matched control subjects when matching birds, boats, cars, chairs or faces. P.S. makes significantly and specifically more errors than normal controls when matching individual faces.
Data Analysis of the Behavioral Experiment
Error rates and RTs for correct responses were analyzed. RTs that were longer than 3 SDs of the mean were discarded. The difference between P.S.'s score and the normal controls' average score divided by the standard deviations of the normals gave a Z-score, which gives a measure of the patient's performance relative to controls (e.g. Dixon et al., 1998). A Z-score > 3 means that P.S.'s performance is above or below 3 SDs of the normals. We also report the analyses using a modified t-test developed to compare a single case to a small sample of normal controls (Crawford and Garthwaite, 2005), avoiding the inflation of type I error rate and exaggeration of the abnormality of patient's score when using the Z-score only (Crawford and Howell, 1998).
Stimuli and Procedures of the Imaging Experiments
Subjects were scanned with a 1.5 T Philips Gyroscan Intera scanner at the University of Louvain, St-Luc Clinic, Brussels (all control subjects and event-related imaging experiment of P.S.) and a 3 T Siemens AG Magnetom Trio at the Donders Center in Nijmegen (one localizer and a pair of runs in the block design experiment for P.S.) provided with standard quadrature birdcage head coils. In each session, a 3D T1-weighted data set encompassing the whole brain was acquired for every subject (110 slices, 1.5 mm slice thickness, matrix size = 256 × 256 × 256). Single shot gradient-echo-planar imaging (EPI) was performed using the BOLD contrast effect as an indirect marker of local neuronal activity (Ogawa et al., 1990).
In experiment 1, 30 5 mm axial slices (TR = 3000 ms, TE = 40 ms, flip angle = 90°, matrix size = 64 × 64, FOV = 250) were acquired. Each run lasted 5 min 33 s (111 TRs). To localize the face-sensitive regions, two or three independent ‘localizer’ scans were run in which subjects viewed alternating blocks of faces, blocks of objects (18 s blocks) and a blank fixation screen (9 s blocks), as in Rossion et al. (2003). During the blocks of faces and objects stimulation, subjects performed a one-back within-category discrimination task, as in previous studies (e.g. Kanwisher et al., 1997). The pairs of localizer scans were repeated three times for P.S. at different recording sessions separated by several months to control for consistency of the results. Following the localizer scans, three scans were acquired using an fMR adaptation design, which consisted of alternating blocks of different faces, different cars, identical faces, identical cars (18 s blocks) and a blank fixation screen (9 s blocks). Each face/object block consisted of 18 (different or identical) stimulus presentations of 800 ms followed by 200 ms blank. Stimuli were grayscale images subtending, on average, ±3° of the visual field, they were matched for mean luminosity and varied location by 20 pixels in x (10%) and 40 pixels in y (13%). A set of 36 different faces (18 males) and 36 different cars was used in total, minimizing the number of repetitions for each picture across epochs and runs (4.5 on average). Since facial identity is known to be processed automatically in the neuronal populations tested (Rolls, 1992; Gauthier et al., 2000), we used an independent detection task in both of our fMRI experiments, as done previously (e.g. Gauthier et al., 2000; Winston et al., 2004; Rotshtein et al., 2005). More precisely, the subject's task was to detect the occurrence of rare face or car stimuli that appeared in red, in a block of grayscale stimuli (colour detection task). There were two or three target trials by epoch, the same number of targets for all conditions on average. Using an independent detection task ensured that subjects were paying attention during the whole experiment, while performing at the same level for all conditions. Furthermore, we used a task that the patient was able to do (Price and Friston, 1999) as well as normal controls, in order to avoid the potential confound that any altered neuronal processing in P.S.'s brain areas could be interpreted as a decrease of general attentional level and/or performance during scanning. Stimuli and blocks were displayed in a pseudo-random order with a PC running E-prime 1.1 (P.S.T, Inc.) through a projector surface located over the head of the subject and viewed with an angled mirror.
In experiment 2, four runs were acquired using an event-related fMR adaptation design in which one trial consistent of a sequentially presented pair of faces or objects that were either identical or different. In each run 21 5 mm axial slices (TR = 1500 ms, TE = 40 ms, flip angle = 90°, matrix size = 4 × 64, FOV = 250) were acquired and runs lasted 5 min. One run contained 32 stimuli pairs (16 identical and 16 different). Stimuli were presented for 500 ms, separated by a 500 ms blank and each pair was followed by a 7500 ms blank fixation screen. One second before each pair the black fixation cross turned red to signal the upcoming stimuli. The second stimulus of each pair was 10% smaller than the first to minimize adaptation effects due to the repetition of the exact same image (Eger et al., 2004). We used a set of 12 colored faces (6 males) and 12 colored chairs, subtending, on average, ±3° of the visual field, and subjects had to detect the occurrence of grayscale stimuli (25% of trials). Pictures of chairs were used as control objects in the event-related experiment because they were found to activate the face-sensitive areas to a lesser extent than cars, making it easier to localize the regions of interest.
Data Analysis of the Imaging Experiments
The fMRI signal in the different conditions was compared using BrainVoyager 2000 (version 4.9, BrainInnovation, Maastricht, The Netherlands) applying a regression analysis. Prior to analysis, preprocessing consisted of linear trend removal, temporal high-pass filtering (removing frequencies lower than 3 cycles/run) and correction of small interscan head movements (Friston et al., 1995). The data were spatially smoothed using a Gaussian filter of 2.8 mm full width at half-maximum (FWHM), and transformed into Talairach space (Talairach and Tournoux, 1988). For anatomical reference, the statistical maps computed were overlaid to the 3D T1-weighted scans. The predictor time courses of the regression model were computed on the basis of a linear model of the relation between neural activity and hemodynamic response, assuming a rectangular neural response during phases of visual stimulation (Boynton et al., 1996).
First, the face-sensitive regions were localized in each individual subject. In experiment 1 the contrast (faces–objects) was computed using the ‘localizer’ runs and all contiguous voxels in the middle fusiform gyrus significant at P < 0.05 (one-tailed, Bonferroni-corrected for multiple comparisons) were considered for further analysis. Given the reduced sensitivity of the event-related experiment compared to the block design fMRI (Mechelli et al., 2003) and because normal subjects performed this experiment at different session than the localizers, we localized the face-sensitive areas using the ‘internal localizer’ (faces–chairs) in the second experiment. Face-sensitive areas were selected by applying the contrast [(identical faces + different faces) – (identical chairs + different chairs)] to the four event-related runs and voxels in the middle fusiform region significant at 0.001 (one-tailed, uncorrected) were considered for further analysis. Second, the above-defined regions of interest (ROI) were tested for fMR adaptation to facial identity with a repeated-measures ANOVA using the contrast: (different faces–identical faces). Third, in order to directly compare P.S. and the control subjects, the percent signal change in the ROI was computed for each condition. In experiment 1 the average percent signal change was calculated over the 18 s stimulation block. In experiment 2, three data points around the peak of the hemodynamic response — defined individually — were averaged to estimate the percent signal change. fMRI signal changes were calculated using the baseline epochs displaying a fixation cross as reference. Fourth, the percent signal change was used to compute two fMR-adaptation indexes for faces (DF–SD and DF–SF/DF+SF) and for objects (DO–SO and DO–SO/DO+SO) for each subject, allowing a comparison between P.S. and the control group in each experiment by means of Z-scores and the modified t-test score of Crawford and Garthwaite (2005).
The object-sensitive region in the right parahippocampal gyrus was determined by the contrast (objects–faces) computed on the ‘localizer’ runs in experiment 1 and by the contrast [(identical chairs + different chairs) – (identical faces + different faces)] in experiment 2. Because this region had a particularly large size (thousands of voxels for some subjects), for adequate comparison with the face-sensitive areas, a cluster of 4 × 4 × 4 voxels located in the center of the region (P < 0.05 corrected for multiple comparisons) was considered for further analysis.
Results
Behavioral Experiment
For discrimination between categories in the 2AFC task, performance was at ceiling for all subjects (mean: 99% including P.S., who made no mistakes). P.S.'s RTs were within the normal range for all conditions of between-category discrimination (Fig. 2A). For the within-category discrimination, all controls subjects were at ceiling for all conditions (>98%), including faces, but the performance was lower for cars (92%). P.S. differed from controls only for the individual discrimination of faces (83%, Z = 9.44, P < 0.001; t = 9.57, P < 0.001; Fig. 1). Her correct RTs for individual discrimination did not differ from controls for all the conditions (Fig. 2B), except for faces (slowing down of 794 ms compared to the control mean, Z = 5.04, P < 0.01; t = 5.09, P = 0.001). When considering the ratio between RTs for individual discrimination and categorical discrimination (i.e. normalizing RTs for each subject), P.S.'s response times were dramatically slower relative to normal controls for faces (Z = 3.97, P < 0.001; t = 3.71, P < 0.05) and did not differ from controls for all other conditions (Zs < 1; ts < 1). These results complement previous evidence showing that P.S.'s deficit lies specifically with the discrimination and recognition of faces at the individual level (Rossion et al., 2003; Caldara et al., 2005).
Performance (response times) in between category and within-category object matching tasks. (a) P.S. responds normally fast in a between-category matching task for faces, birds, boats, cars and chairs. (b) P.S. responds abnormally slow in a within-category face matching task. (c) RTs of correct responses in a within-category matching task normalized by the corresponding RTs in a between-category matching task [(RTs within – RTs between)/(RTs within + RTs between)]. P.S. is significantly and selectively slower than control subjects in the within-category face matching condition.
Imaging Experiment 1: Recovery from fMR Adaptation in a Block Design
Behavioral Data During Scanning
In the localizer experiment, subjects performed the one-back task at ceiling (mean accuracy 99 ± 0.6%, P.S. 94.3%). P.S. was slower than both young and age-matched controls (mean RT: young 416 ± 31ms, age-matched 395 ± 15 ms, P.S. 503 ms; P < 0.05). Whereas controls performed equally well and fast for objects and faces, P.S.'s performance for the blocks of objects was significantly better than for faces (16.5 versus 1 error; P < 0.001) and she tended also to be faster for objects than faces (486 versus 537 ms; t = 0.07).
In the block-adaptation experiment, the color detection task was performed at ceiling for both controls and P.S. in all four conditions (DF: 93.9 ± 2.3 versus 94.4; SF: 98.9 ± 1.7 versus 99.5; DO: 98.5 ± 1.6 versus 100; SO: 98.0 ± 2.8 versus 98.8; all Ps > 0.1, NS). The controls and P.S. also responded with similar speed in the four conditions (DF: 492 ± 52 versus 460; SF: 449 ± 46 versus 468; DO: 462 ± 34 versus 506; SO: 449 ± 48 versus 481; all Ps > 0.1).
Neuroimaging Results
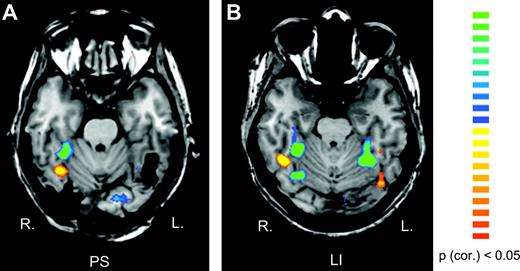
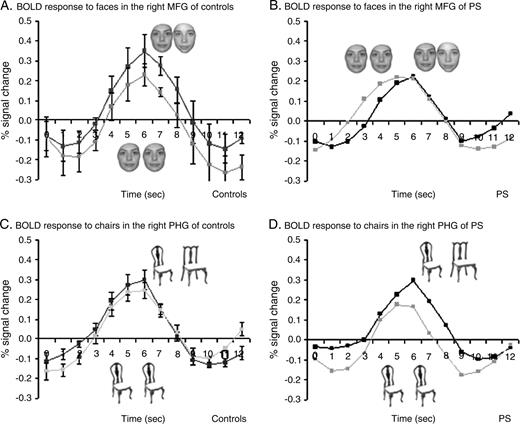
When comparing blocks of faces and objects in the ‘localizer’ paradigm (Kanwisher et al., 1997), P.S. and all control subjects showed activation in the rMFG (P < 0.05 corrected for multiple comparisons, see Fig. 3A,B). The center of activation was located in the same region in P.S. (36, −54, −20; size: 576 voxels) as in normal controls (37 ± 6, −47 ± 9, −18 ± 3; size: 545 ± 472 voxels), confirming previous observations (Rossion et al., 2003). All control subjects also had a significant activation in the rIOG (36 ± 6, −76 ± 10, −10 ± 6; size: 295 ± 365 voxels) when comparing blocks of faces and objects, whereas this region was structurally damaged in P.S. (Fig. 3A,B). Additionally, normal subjects also showed activation in response to faces in the left MFG (–41 ± 6, −49 ± 9, −16 ± 11; size: 424 ± 230 voxels), again in an area that was damaged in P.S.'s cortex (Fig. 3).
Regions of interest in the right MGF and in the right PHG in P.S. (a) and in one control subject (b). The color scale represents statistical values comparing the fMRI signal while subjects viewed blocks of faces versus blocks of objects. Yellow-red regions yield larger BOLD signal in response to faces than other objects and green-blue regions respond more to objects than faces. The right MGF (faces versus objects) and PHG (objects versus faces) served as ROI for analyzing the block-design data.
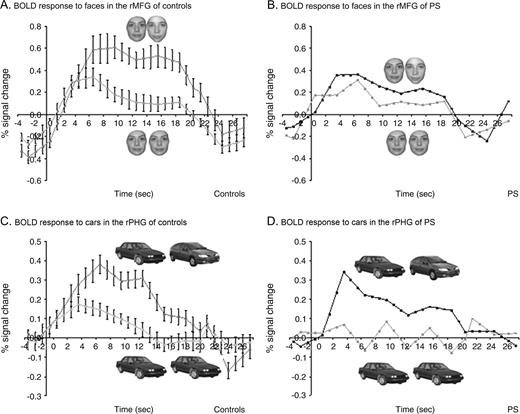
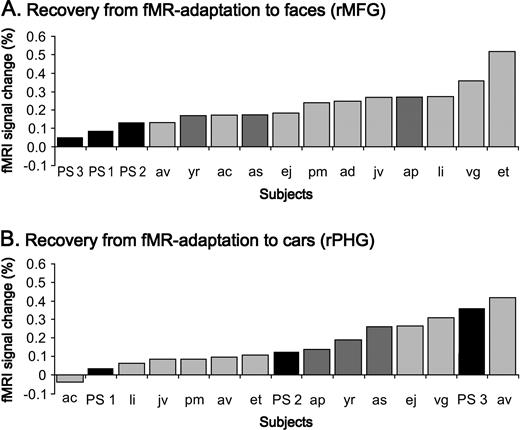
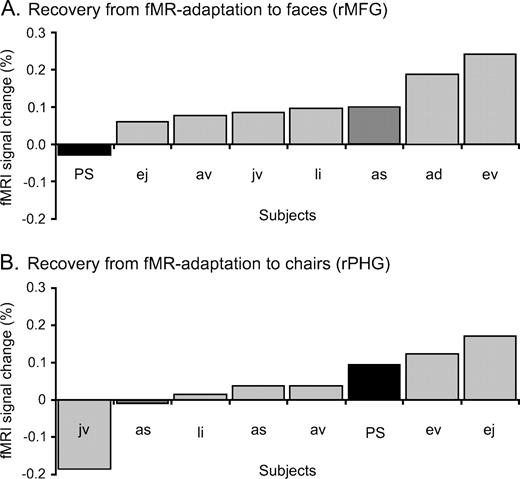
In the group analysis on normal subjects, the expected recovery from fMR adaptation to facial identity was highly significant in the rMFG (paired t-test P < 0.001). In addition, in every single control subject there was a higher activation level in response to blocks of different faces than during blocks of identical faces. The contrast ‘different faces–same faces’ (DF–SF) was significant at P < 0.05 in 11/12 normal subjects and showed a non-significant trend in the predicted direction at P < 0.139 in the remaining subject. In this latter subject the same contrast was highly significant (P < 0.001) in the lMFG. In contrast, the recovery from fMR adaptation in this block design appeared to be reduced in P.S.'s structurally intact rMFG. While blocks of identical faces yielded similar response profiles in the rMFG of P.S. and controls (see Fig. 4B), the average signal during blocks of different faces was almost the double (1.7 times higher) in controls than in P.S. (see figures 4A and 4B). The index of recovery from adaptation (DF-SF) was lower for P.S. than for every individual control subject (see Fig. 5A) and it was significantly smaller in P.S. than in the three age-matched control subjects taken as a group (Z = 2.04, P < 0.05; t = −1.7, P < 0.1). There was also a non-significant trend in the same direction when comparing the ratio (DF–SF)/(DF+SF) for P.S. and the age-matched controls (Z = 1.31, P = 0.09; t = −1.1, P = 0.19). Moreover, for P.S. the contrast DF–SF was not significant in two of the three scanning sessions (P < 0.463, P < 0.375 and P < 0.013).
Recovery from fMR adaptation in the right MFG and PHG in experiment 1 (block design). (a) Normal recovery from fMR adaptation to facial identity in the right MFG of control subjects (n = 12; three runs averaged for each subject) contrasts with (b) reduced recovery from fMR adaptation in the rMGF of P.S. The average percent signal change (±SE) from baseline fixation is plotted for the identical and the different face conditions. Stimulus presentation lasted for 18 s. While the response to blocks of identical faces yielded a similar response in P.S. as in control subjects, the response to different faces increased on average by 0.25% (SE 0.04) in controls, contrasting with a strongly reduced 0.09% increase in P.S. (c) Normal recovery from fMR adaptation to car identity in the right PHG of control subjects (n = 12) and (d) P.S.
Reduced recovery from fMR adaptation to facial identity in the rMFG of the prosopagnosic patient P.S. contrasts with a normal recovery from fMR adaptation in her rPHG (in experiment 1). (a) The difference in percent signal change between DF and SF (DF–SF) is plotted for P.S. (black bars) and each individual control subject (grey bars) in an increasing order. P.S. has a significantly reduced difference in fMRI signal change compared to the three age-matched control subjects (dark grey bars), for all three scanning sessions performed on her (three times; three runs averaged). (b) In the right PHG, however, P.S. shows a completely normal recovery from fMR adaptation to the identity of cars, as indicated by the distribution of her three measurements in a plot ranking the differences in percent signal change between DO and SO.
In the rMFG we also observed a significant recovery from fMR adaptation for cars in normal subjects in the group analysis (paired t-test, P < 0.05). This recovery from adaptation was not significantly larger in the controls than in P.S. (DO–SO: Z = 0.15, P = 0.44; t = −0.16, P = 0.445) and (DO–SO/SO+SO: Z = −0.57, P = 0.28; t = -0.002, P = 0.5).
Located medially to the rMFG, a region in the right parahippocampal gyrus (rPHG) (24 ± 5, −53 ± 9, −12 ± 3) showed higher BOLD signal for objects than for faces in the ‘localizer’ scans (Epstein and Kanwisher, 1998). In this object-sensitive region, there was also a recovery from fMR adaptation to object (i.e. cars) identity that reached significance in the group analysis (P < 001), and in 5/12 control subjects (P < 0.05). In striking contrast to the abnormal recovery from fMR adaptation in the more lateral face-sensitive fusiform region, P.S. showed a normal difference in percent signal change between different objects (DO) and same objects (SO) in this region (see Fig. 4C,D). Indeed, the difference in percent signal change between DO and SO was in the same range for P.S. (0.172) and normal controls (mean 0.164, SE 0.4) (see Fig. 5B). Both indexes (DO–SO) and (DO–SO/DO+SO) were not significantly different in P.S. and the control subjects (DO–SO: Z = −0.06, P = 0.48; t = 0.07, P = 0.47) and (DO–SO/SO+SO: Z = −0.3, P = 0.38; t = 0.29, P = 0.40).
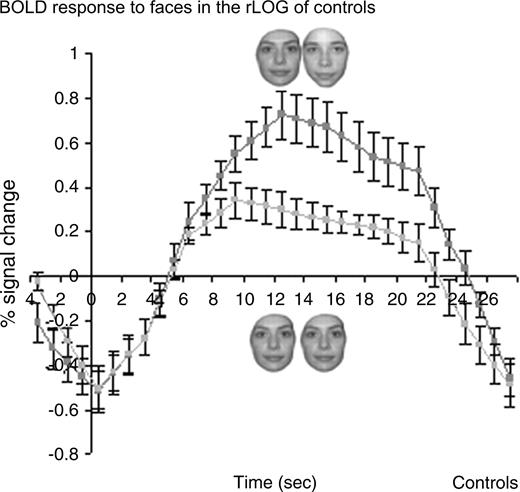
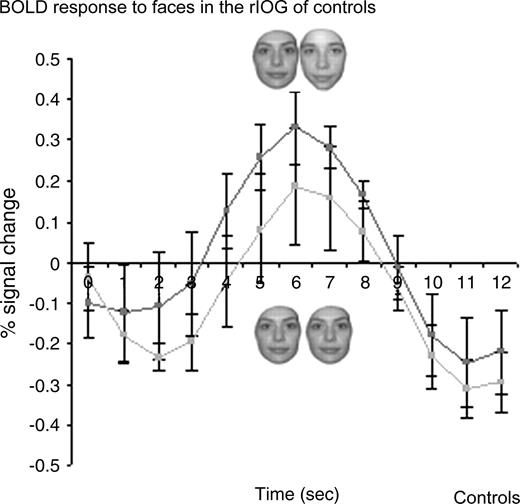
Finally, we analyzed the recovery from adaptation profiles in the rIOG for normal subjects, a cortical region that is structurally damaged in P.S. In the group analysis, we observed a significant recovery from fMR adaptation to facial identity in this face-sensitive occipital region (paired t-test, P < 0.01) (Fig. 6). Furthermore the activation level in response to blocks of different faces was higher than during blocks of identical faces in 11/12 subjects and the contrast (DF–SF) was significant at P < 0.05 in 10 control subjects.
Recovery from fMR adaptation to individual faces in the right IOG of normal controls in experiment 1 (block design). A significant recovery from fMR adaptation to facial identity is observed in the right IOG of control subjects (n = 12; three runs averaged for each subject), a region that is structurally damaged in P.S.
Imaging Experiment 2: Recovery from fMR Adaptation in an Event-related Design
Behavioral Data During Scanning
In the event-related adaptation experiment, the color detection task was performed at ceiling for both P.S. and controls in all four conditions (DF: 97.5 versus 99.2 ± 1.9; SF: 96.3 versus 99.2 ± 1.9; DO: 95.0 versus 92.5 ± 8.0; SO: 98.8 versus 92.5 ± 8; all Ps > 0.1). P.S. and the controls also responded with similar speed in the four conditions (DF: 625 versus 749 ± 213; SF: 651 versus 543 ± 36; DO: 838 versus 722 ± 171; SO: 759.0 versus 616 ± 205; all Ps > 0.1).
Neuroimaging Results
The results of the event-related experiment largely confirmed and extended the abnormal profile of facial identity coding in the rMFG of P.S.. Whereas normal controls showed a large recovery from fMR adaptation for trials presenting pairs of different faces (paired t-test, P < 0.001) in the rMFG (41 ± 6, −47 ± 10, −17 ± 5; size: 534 ± 474 voxels), there was no evidence of such recovery for P.S., as illustrated in Figure 7A. In the face-sensitive region of the rMFG (34, −56, −21; size: 133 voxels) her BOLD response to pairs of different faces (0.18% signal change) was similar to her BOLD response to pairs of identical faces (0.21% signal change) (see figure 7B). The contrast comparing different and same face trials was not significant (P < 0.68) in P.S., whereas this contrast was significant in 3/7 control subjects (P < 0.05) and showed a non-significant expected trend in the predicted direction in the remaining control subjects (P < 0.06, P < 0.07, P < 0.09, P < 0.14). Moreover, in the latter subjects the contrast DF–SF was significant (P < 0.05) in the corresponding left middle fusiform region. The difference in percent signal change between pairs of different and identical faces (DF–SF) was much smaller in P.S. (–0.029) than in the group of control subjects (mean: 0.12, SE 0.03; Z = 2.26 P < 0.05; t = −2,0, P < 0,05) (Fig. 8A). Likewise, the ratio (DF–SF/DF+SF) was significantly larger in the controls than in P.S. (Z = 3.8, P < 0.001; t = −3.51, P < 0.01). Contrasting with her abnormally weak BOLD response to pairs of different faces, P.S. had a normal response to pairs of identical faces as illustrated in Figure 8B. Confirming again the results of the block experiment, the BOLD signal peaks in the SF trials were very similar in P.S. (0.21% signal change) and normal controls (mean 0.23% signal change, SE 0.23) in terms of the peak height (see Fig. 7B).
Recovery from fMR adaptation in the right MFG and PHG in experiment 2 (event-related design). (a) The average percent signal change (±SE) from baseline fixation is plotted for the ‘different faces’ and the ‘same faces’ conditions in controls (n = 7) and (b) in P.S. Reduced response in the rMFG of P.S. to trials with different faces reveal an abnormal neuronal processing of facial identity in the rMFG of the prosopagnosic patient. Trial starts at time = 0 s. The event-related BOLD response in trials with identical faces was normal in P.S. compared to the controls. Note that the event-related hemodynamic response appears to start and peak earlier in this condition for P.S. as compared to the group of controls, but this was not systematic, i.e. observed in only half of the subjects. The average percent signal change (±SE) in the rPHG to trials with different and identical chairs did not differ significantly in (c) normal controls and (d) P.S.
P.S. shows significantly reduced recovery from fMR adaptation to facial identity in the rMFG, but normal recovery from fMR adaptation in the rPHG in experiment 2 (event-related design). (a) The difference in percent signal change between DF trials and SF trials is plotted for P.S. and each individual control subject in an increasing order. P.S. (black bars) has a significantly reduced difference in fMRI signal change compared to the age-matched-control (dark grey bars) and the remaining control subjects in the rMFG. (b) In the rPHG P.S. shows a normal recovery from fMR adaptation to the identity of cars.
In the event-related design, we did not observe a significant recovery from fMR adaptation for objects (i.e. chairs) in the rMFG in normal subjects (paired t-test P = 0.36). And the response pattern in P.S. did not differ from this result, as indicated by both fMR adaptation indexes (DO–SO: Z = −0.94, P = 0.17; t = 0.84, P = 0.22) and (DO–SO/SO+SO: Z = −0.54, P = 0.29; t = 0.44, P = 0.34).
In the object-sensitive region in the rPHG (29 ± 4, −44 ± 11, −13 ± 5), P.S. showed the strongest trend for recovery from adaptation of all subjects (P < 0.07) for the contrast DO–SO. On average, her difference in percent signal change between DO and SO (0.09) was larger than in normal controls (mean 0.03, SE 0.04) (Fig. 8B), but this difference was not significant (DO–SO: Z = −0.59, P = 0.28; t = 0.51, P = 0.31) and neither was the same comparison using the ratio (DO–SO/DO+SO) (Z = −0.95, P = 0.17; t = 0.89, P = 0.20).
Lastly, we focused on the rIOG (44 ± 5, −67 ± 7, −17 ± 7; size 135 ± 105 voxels) of normal subjects, a region which is structurally damaged in P.S. and found a strong recovery from adaptation in response to faces in the group analysis (paired t-test P < 0.01) (Fig. 9). In this occipital region, pairs of different faces yielded higher activation levels than pairs of identical faces in 6/7 subjects and this comparison (DF–SF) was significant in four controls.
Recovery from fMR adaptation to individual faces in the rIOG of normal controls in experiment 2 (event-related design). A significant recovery from fMR adaptation to facial identity is observed in the rIOG of control subjects, a region that is structurally damaged in P.S.
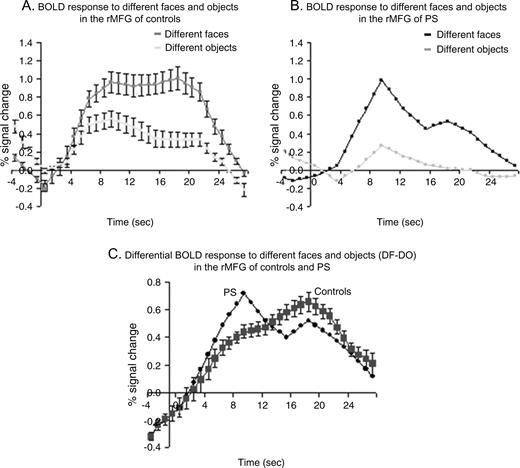
The differential BOLD response to faces versus objects in the rMFG of P.S. is not sustained throughout the second half of the stimulus presentation block, contrary to the undiminished signal in normal controls. (a) The average percent signal change (±SE) from baseline fixation is plotted for the ‘different faces’ and ‘different objects’ conditions of the localizer experiment in controls (n = 12) and (b) in P.S. (c) When the block is divided into two parts (1–9 s and 10–18 s) it appears that the differential response is higher in P.S. than controls during the initial half and lower during the second part of the 18 s stimulation block.
Complementary Analyses for the Localizer Experiment
In the localizer scans (used to identify the face-sensitive regions in the ventral visual pathway) blocks of different faces (DF) are contrasted with blocks of different objects (DO), while subjects perform a one-back discrimination task. In the two fMR adaptation experiments, on the contrary, blocks (or pairs) of different and identical faces are compared, while subjects are performing an independent color detection task. Given that we observed a reduced or absent recovery from adaptation to different face stimuli in the rMFG of P.S. in the two latter experiments, we also analyzed the profile of the hemodynamic response to different faces and objects in the face localizer experiment for P.S. and the normal control subjects.
Overall, the average percent signal change difference between faces and objects did not differ between P.S. (DF–DO: 0.43 ± 0.15) and the normal controls (DF–DO: 0.39 ± 0.05) (Z = −0.93, P = 0.18; t = 0.77, P = 0.23), confirming our previous findings (Rossion et al., 2003). However, when computing separately the difference in percent signal change between faces and objects for the first half of the block (1–9 s) and the second half of the block (10–18 s), P.S. differed significantly from the control subjects (Fig. 10A,B). During the first half of the block the differential response was higher in P.S. (DF–DO: 0.36) than in controls (DF–DO: 0.22 ± 0.07) (Z = −2.09, P < 0.05; t = 1.92, P < 0.05). However, this activation level was not sustained and was significantly lower than in the controls during the second half of the block (DF–DO: P.S. 0.5 versus controls 0.55 ± 0.03) (Z = 1.83, P < 0.05; t = −1.60, P < 0.07) (Fig. 10C). Thus, while we confirmed our previous findings of an overall normal range response for faces in the rMFG of patient P.S. during the localizer scans, her profile of activation indicates a lack of sustained responses to different faces, in line with the results of the adaptation experiments reported above. Note that P.S.'s normal mean response in the rMFG to the block of different faces is largely due to the fact that the initial response of the patient in the localizer scans is in the upper range and compensates for the significantly lower response found in the second part of the epoch (Fig. 10a).
It should be kept in mind, however, that this result in the localizer scans was obtained during a one-back discrimination task that P.S. was unable to perform correctly. It is of particular interest that the same profile of response is found in the adaptation experiments in which P.S. performed an independent task as well as controls and in which we had a measure of the recovery from adaptation by comparing to blocks or trials of identical faces repeated.
Although the present design did not directly address this question, it provides indications that P.S. does not have an intrinsic reduction of signal for faces (versus objects) as compared to controls in the right fusiform gyrus. Indeed, P.S. is in the normal range if one compares the percent signal change when the same face and the same object are repeated (SF–SO) (experiment 1 — block design: P.S. 0.08% versus controls 0.13 ± 0.16%, Z-score 0.35, P = 0.36; experiment 2 — event-related design: P.S. 0.22% versus controls 0.17 ± 0.21%, Z-score −0.22, P = 0.41). In other words, when presented always the same stimulus, P.S. shows normal face selectivity.
Discussion
The neuroimaging experiments reported here reveal that the rMFG of P.S. — a patient with acquired prosopagnosia following brain damage — presents an anomalous response pattern with respect to individual face discrimination, despite being structurally intact and responding as well as in normal subjects to faces at the basic category level. The abnormal signal in P.S.'s right fusiform gyrus most likely reflects a failure of recovery to adaptation to different facial identities, because in the patient the BOLD response to both identical and distinct faces is at the level of the response to identical faces in normal control subjects. The reduced response observed in the ‘different face’ conditions in P.S.'s rMFG contrasts sharply with the recovery from fMR adaptation to facial identity occurring in the corresponding area in normal control subjects, as observed previously (Gauthier et al., 2000; Henson et al., 2000; Grill-Spector and Malach, 2001; Eger et al., 2004; Winston et al., 2004; Rotshtein et al., 2005). It also contrasts with the normal recovery from fMR adaptation to objects (i.e. cars and chairs) observed in the patient's object-sensitive region in the rPHG (Epstein et al., 1999), showing that the lack of recovery from adaptation is not unspecific. [Contrary to the robust adaptation to faces, words and houses in the rPHG reported previously by Avidan et al. (2002), the adaptation to chairs in the PHG did not reach significance in our study. The reduced sensitivity of the present event-related fMRI experiment compared to the block design used by the previous authors might account for our failure to observe adaptation in response to chairs in the rPHG (Mechelli et al., 2003).] The present experiment did not allow us to test the recovery from adaptation to non-face objects in the lateral occipital complex (LOC), defined as a region that responds more to objects than scrambled images of objects (Malach et al., 1995). This area does not appear to present a larger response to face than non-face object categories, but shows adaptation to shape repetition (Grill-Spector et al., 1999; Kourtzi and Kanwisher, 2000; Grill-Spector and Malach, 2001). An interesting extension of this work would thus be to test whether the LOC, which appears to be functionally preserved bilaterally in the patient's brain (Sorger et al., 2004), shows normal recovery from adaptation to objects, including faces. This area may contribute to the normal within-category discrimination of non-face objects, but also to some extent to the reduced — but not abolished — ability of the patient to discriminate faces at the individual level.
The specific alteration of the neuronal response to facial identity is in line with P.S.'s behavioural impairments. In a 2AFC matching task P.S. correctly and rapidly performed between-category discriminations for all object categories tested. [Admittedly, between-category discrimination (e.g. discriminating a car from a boat) is, almost by definition, a very simple task. It may be harder only for objects with similar shapes, belonging to the same superordinate categories, such as fruits for instance. We have shown previously that PS was able not only to discriminate, but to name accurately and quickly all the objects (including all fruits, animals) of the Snodgrass and Vanderwart's databank (see Rossion et al., 2003). Moreover, even if the performance was at ceiling, we measured RTs and subjects were instructed to respond as fast as possible. P.S.'s RTs in this between-category discrimination task did not differ from controls.] She also discriminated all objects (i.e. cars, chairs, boats, birds) at the individual level, except faces. Thus she presents an abnormal response pattern specifically with respect to individual face discrimination, both at the behavioural and the neuronal level.
Cases of prosopagnosia described with a deficit restricted to the category of faces are extremely rare (Sergent and Signoret, 1992) and most patients have associated deficits at the basic level for object recognition (e.g. Damasio et al., 1982; Sergent and Signoret, 1992; Clarke et al., 1997; Dixon et al., 1998; Gauthier et al., 1999), including the notorious prosopagnosic patient L.H. described by Farah and colleagues (Farah et al., 1995; see Levine and Calvanio, 1989). Furthermore, these patients are generally found to be impaired at subordinate judgments of non-face categories, especially when tested in fine discrimination tasks and/or measuring RTs as well as recognition performance (Damasio et al., 1982; Gauthier et al., 1999; Laeng and Caviness, 2001). Here, P.S. presents a normal object recognition performance at the basic level, as also shown by her flawless recognition of the whole set of colorized Snodgrass–Vanderwart pictures (see Rossion et al., 2003; Table 3). Moreover, she is able to discriminate non-face categories at the individual level as accurately and as fast as normal controls, even though, as noted in the Introduction, she may show response biases and be slightly slowed down in ‘same/different’ tasks during within-category discrimination tasks on non-face stimuli compared to normal controls (Rossion et al., 2003). Such response biases and slowing down are common in brain-damaged patients, particularly when task difficulty increases (e.g. Benton, 1977, 1986; Gauthier et al., 1999). However, overall, her performance in computer object discrimination and recognition tasks indicates that, unlike most cases of acquired prosopagnosia (e.g. Damasio et al., 1982; Levine and Calvanio, 1989; Sergent and Signoret, 1992; Gauthier et al., 1999), P.S.'s ability to recognize and discriminate non-face objects is remarkably preserved. Her deficit appears to be restricted to the extraction of diagnostic information on faces (Caldara et al., 2005), most likely following damage to a neural subsystem for faces developed through extensive visual experience (Morton and Johnson, 1991; Le Grand et al., 2001).
Neuroimaging studies in normals have consistently shown the strongest activation in the right fusiform gyrus, both for face detection and individualization (Gauthier et al., 2000; Grill-Spector and Malach, 2001; Eger et al., 2004; Grill-Spector et al., 2004; Winston et al., 2004; Rotshtein et al., 2005). The anomalous BOLD response to conditions with different faces in the rMFG of the prosopagnosic patient P.S. reported here points towards a critical function of this region in individual face discrimination. Contrary to the abnormally weak BOLD signal yielded by the presentation of different facial identities in the rMFG, as also found in the second time epoch of her localizer scans, the response to conditions with identical faces in the same region in P.S. is similar to that of control subjects. This observation is in accordance with P.S.'s preserved ability to categorize faces at the basic level, despite her severe and selective impairment in discriminating faces at the individual level. We suggest that this normal BOLD response in the rMFG to ‘identical’ conditions underlies her conserved face detection skill and conclude that activation in the rMFG is sufficient for face detection. This proposal is consistent with recent evidence showing that faces that are classified at the basic category level activate the face-sensitive region in the fusiform gyrus, even without being identified at the subordinate level (Grill-Spector et al., 2004). Additionally, our data support the view that the initial input to the rMFG cannot stem exclusively from the rIOG (Rossion et al., 2003). In P.S.'s brain, the inferior occipital cortex is damaged, yet the rMFG yields a normal activation level in response to faces as a category. The input giving rise to this activation must therefore originate from posterior, low-level visual areas other than the rIOG (Kim et al., 2004).
The evidence that face-sensitive neurons in the fusiform gyrus code both the global shape of the category ‘face’ and the fine characteristics of the identity of individual faces in normal healthy adults (Halgren et al., 1999; Gauthier et al., 2000; Grill-Spector and Malach, 2001; Eger et al., 2004; Grill-Spector et al., 2004; Winston et al., 2004; Rotshtein et al., 2005) is in accordance with information analysis of single-cell populations in the monkey brain, showing that the very same neuronal population can subserve the two functions (Rolls, 1992; Rolls and Deco, 2001), perhaps at different time-scales (Sugase et al., 1999). To our knowledge, the present fMR adaptation experiment is the first report of a neural dissociation between these two functions in acquired prosopagnosia. It indicates that an area like the rMFG can be necessary but not sufficient for a complex function like face individuation, while being sufficient but not necessary for a simpler task like face detection.
P.S.'s prosopagnosic deficit is most likely due to the massive damage encompassing the rIOG, the same region in which the largest overlap of lesions causing prosopagnosia is found (Bouvier and Engel, 2004). However, prosopagnosia can also follow after more anterior lesions, i.e. to the fusiform gyrus, sparing the rIOG (e.g. Barton et al., 2002; Delvenne et al., 2004). These observations indicate that successful face identification requires both the rMFG and the rIOG to be structurally and functionally intact. In contrast, the distinction of faces from other categories, or the segmentation of a face stimulus in a visual scene, can be preserved despite lesions to at least one of these two areas.
The reduced BOLD signal in the ‘different faces’ condition in P.S.'s structurally intact right fusiform gyrus reveals an ineffective coding of facial identity in this face-sensitive visual area. This abnormal response in the spared visual system of a patient with acquired prosopagnosia contrasts with the recovery from fMR adaptation observed in the same region in normal adults. The physiological mechanisms underlying the recovery from adaptation effects are not completely understood, and may be plural (see Grill-Spector and Malach, 2001; Henson, 2003). Regarding the representation of faces, different facial identities may be coded by partially overlapping or degenerate (Tononi et al., 1999) subsets of neurons in a relatively small population (Rolls, 1992; Young and Yamane, 1992) in the extrastriate visual cortex. According to this view (see Rolls and Deco, 2002), the recurring presentation of identical faces repeatedly activates the subpopulation of cells coding for this identity, leading to a decreased neuronal discharge at the global level that can be recorded in the fMRI hemodynamic response (the so-called fMR adaptation) (Gauthier et al., 2000; Grill-Spector and Malach, 2001; Henson, 2003; Winston et al., 2004). In contrast, the presentation of different facial identities activates a partially distinct neuronal response pattern for each identity in the same population, leading to a larger global BOLD response (recovery from adaptation, see Grill-Spector and Malach, 2001). The failed recovery from fMR adaptation to facial identity shown here in P.S.'s rMFG mirrors a lack of these distinct sparse response codes representing the different facial identities in the rMFG, possibly due to missing (re)entrant input from the damaged rIOG.
Addressing the question of the nature of face representations in the two areas discussed here, a recent fMRI study in normal subjects has shown that fMR adaptation to familiar faces in the rIOG reflects the physical difference between morphed faces, whereas the rMFG shows no sensitivity to the physical difference as long as the face stimuli are perceived as similar identities (Rotshtein et al., 2005). However, the rMFG did show release from adaptation when stimuli were perceived as different identities (Rotshtein et al., 2005; see also Eger et al., 2004). Even though familiar and unfamiliar faces are processed differently in these areas, as we have shown previously using morphs between familiar and unfamiliar faces (Rossion et al., 2001), our current results are consistent with and complement the above-mentioned observations. During the blocks or trials of different face stimuli, we presented clearly distinct facial identities (corresponding to the ‘between’ condition in Rotshtein et al.'s experiment), thus expecting to observe a recovery from adaptation in both areas in normal controls, as found previously (Gauthier et al., 2000; Grill-Spector and Malach, 2001; Eger et al., 2004; Grill-Spector et al., 2004). For the patient P.S., the faces that are of different identities are perceived as identical in a large number of cases, hence the reduced recovery from adaptation in her rMFG, consistent with the observations of Rotshtein et al. (2005). However, in this latter study, only normal controls were tested and thus the question of whether the rIOG was necessary for intact face discrimination could not be addressed. Furthermore, in normal subjects, Rotshtein et al. (2005) have not shown, and not claimed, that the rMFG was independent of the rIOG in extracting facial identity. In fact, this latter study suggests that the rIOG is involved (and the present study suggests that it is necessary) for fine-grained discrimination of individual faces, whereas the rMFG appears to extract a more global (perhaps ‘holistic’, see Rossion et al., 2000) representation of identity. All in all, our results indicate that when it comes to differentiating faces, both the rMFG and the rIOG are critical, the rMFG appearing to be dependent on normal sustained inputs from the rIOG. If the rIOG is unable to detect fine physical differences, it may be that the rMFG is no longer able to categorize different facial identities, independent of their physical difference. This leads to the interesting prediction that given her rIOG lesion, in P.S., we should not find a larger recovery from adaptation in the rMFG in the condition ‘between’ of Rotshtein et al. (2005) compared to their condition ‘within’, even in a block design (e.g. alternating between several pairs of faces).
Both the neuroimaging data of P.S. and the significant recovery from adaptation found in normal controls in the rMFG and rIOG in the present study suggest that efficient individual discrimination of faces has to rely on the integrity, and possibly the functional integration (Price and Friston, 2002), taking place between the rMFG and rIOG. Based on the present data (see also Rossion et al., 2003), we suggest that the initial input to the rMFG leading to face-related activation is independent from face-sensitive responses in the rIOG and may originate from posterior, low-level visual areas (Kim et al., 2004). However, in the intact brain, the rIOG is critical to face individualization, perhaps by entertaining a re-entrant loop with the more anterior rMFG. The higher-order, face-sensitive visual area in the rMFG may contribute through re-entrant signalling to the emergence of functional responses in the earlier face-sensitive visual area in the rIOG (Bullier et al., 2001; Galuske et al., 2002), where global information could serve as a header to set up the processing of fine information related to facial identity (Sugase et al., 1999). This proposal is in agreement with the presence of cortical feedback (Mumford, 1992; Lamme and Roelfsema, 2000; Bullier et al., 2001) and re-entrant phasic signaling in the visual cortex (Edelman, 1993). Through these feedback connections, higher-level perceptual computations and representations that involve high-resolution details, fine geometry and spatial precision may involve lower visual areas and be reflected in the later part of their neurons' activities (Mumford, 1992; Lee et al., 1998;). Given their smaller receptive fields, neurons in the rIOG may be fine-tuned to subserve such fine discrimination, which are critical in real life situations (e.g. recognizing the same identity across age differences, changes in lighting, discriminating siblings or twins, etc.). In sum, in our view, the damage to the rIOG underlies P.S.'s prosopagnosic deficit, both directly (i.e. through a damage to the representations and processes taking place normally in this area), but also indirectly, given that this region cannot provide normal inputs to other areas, such as the rMFG.
In the present study, we have reported a functional neuroanatomy study of a single case of prosopagnosia to address the question of the necessary face-sensitive regions coding for facial identity in the human brain and to draw hypotheses regarding their interaction during normal face processing. Our single-case study should by no means be generalized to all cases of prosopagnosia, i.e. that all such patients should present an absence of recovery from adaptation in the rMFG if it is structurally intact. There is considerable variability between acquired prosopagnosics with respect to lesion localization and extent and performance on various discrimination and recognition tasks (e.g. Sergent and Signoret, 1992; Barton et al., 2002) and the cause of the deficit will vary between different patients, preventing such generalization. Recently, there has also been a growing interest in studying cases of developmental or congenital prosopagnosia, i.e. people presenting a deficit in face processing that is apparent from early childhood, without any underlying neurological basis (for reviews see Kress and Daum, 2003; Behrmann and Avidan, 2005). With respect to the findings of the present study, it is interesting to note that cases of congenital prosopagnosia appear to show a normal face-related activation in the rMFG and rIOG, as well as normal recovery from adaptation in these regions (Avidan et al., 2005). Thus, in these cases, there is no correlation between behavioral performance (impaired) in face discrimination and hemodynamic responses (normal) in the intact face-sensitive areas of the right hemisphere, unlike the pattern reported here in the case of a brain-damaged patient. Further research will be needed to determine the neural basis of congenital prosopagnosia, in particular how their behavioral impairments can be related (or not) to an inefficient coding of individual face representations in the right ventral pathway.
In summary, the present study revealed a reduced recovery from fMR adaptation specific to faces in the structurally intact rMFG of a patient with pure prosopagnosia following damage to the right occipital gyrus. These finding supports the view that both the rMFG and the rIOG are critical for coding faces at the individual level. In addition, they show that two levels of processing, namely face detection and individual face discrimination, can be dissociated (i.e. normal and impaired respectively) in the same cortical area, here the rMFG, in line with the behavior of the brain-damaged prosopagnosic patient.
We are very grateful to P.S. for her patience and motivation during the experiments, and to three anonymous reviewers for helpful and constructive comments on a previous version of this manuscript. This study and the first author are supported by a grant (ARC 01/06-267, Communauté Française de Belgique — Actions de Recherche Concertées). B.R. is supported by the Belgian National Foundation for Scientific Research (FNRS).
References
Avidan G, Hasson U, Hendler T, Zohary E, Malach R (
Avidan, G, Hasson, U, Malach, R, Behrmann M (
Barton JJ, Press DZ, Keenan JP, O'Connor M (
Behrmann M, Avidan, G (
Benton AL (
Bouvier SE, Engel SA (
Boynton GM, Engel SA, Glover GH, Heeger DJ (
Brodtmann A, Puce A, Syngeniotis A, Darby D, Donnan G (
Bullier J, Hupe JM, James AC, Girard P (
Caldara R, Schyns P, Mayer E, Smith ML, Gosselin F, Rossion B (
Clarke S, Lindemann A, Maeder P, Borruat FX, Assal G (
Crawford JR, Garthwaite PH (
Crawford JR, Howell DC (
Damasio AR, Damasio H, Van Hoesen GW (
Delvenne JF, Seron X, Coyette F, Rossion B (
Dixon MJ, Bub DN, Arguin M (
Edelman GM (
Eger E, Schyns PG, Kleinschmidt A (
Epstein R, Kanwisher N (
Epstein R, Harris A, Stanley D, Kanwisher N (
Farah MJ (
Farah, MJ, Levinson, KL, Klein, KL (
Friston KJ, Frith CD, Turner R, Frackowiak RS (
Galuske RA, Schmidt KE, Goebel R, Lomber SG, Payne BR (
Gauthier I, Behrmann M, Tarr MJ (
Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW (
Grill-Spector K, Malach R (
Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R (
Grill-Spector K, Knouf N, Kanwisher N (
Halgren E, Dale AM, Sereno MI, Tootell RB, Marinkovic K, Rosen BR (
Haxby JV, Hoffman EA, Gobbini IA (
Henson R, Shallice T, Dolan R (
Ishai A, Ungerleider LG, Martin A, Haxby JV (
Kanwisher N, McDermott J, Chun MM (
Kim M, Ducros M, Carlson T, He S, Ugurbil K, Kim D-S (
Kourtzi Z, Kanwisher N (
Laeng B, Caviness VS (
Lamme VA, Roelfsema PR (
Landis T, Regard M, Bliestle A, Kleihues P (
Le Grand R, Mondloch CJ, Maurer D, Brent HP (
Lee TS, Mumford D, Romero R, Lamme VA (
Levine DN, Calvanio R (
Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TL, Rosen BR, Tootell RB (
Mayer E, Fistarol P, Valenza N (
Mechelli A, Price CJ, Henson RN, Friston KJ (
Morton J, Johnson MH (
Mumford D (
Ogawa S, Lee TM, Kay AR, Tank DW (
Oldfield RC (
Price CJ, Friston KJ (
Rolls ET (
Rossion B, Dricot L, De Volder A, Bodart J-M, Crommelinck M, de Gelder B., Zoontjes R (
Rossion B, Schiltz C, Robaye L, Pirenne D, Crommelinck M (
Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E (
Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ (
Sergent J, Signoret JL (
Sergent J, Ohta S, MacDonald B (
Sorger B, Schiltz C, Caldara R' Kriegeskorte N, Mayer E, Rossion B, Goebel R (
Sugase Y, Yamane S, Ueno S, Kawano K (
Talairach G, Tournoux P (
Tanaka JW (
Tononi G, Sporns O, Edelman GM (
Uttner I, Bliem H, Danek A (
Winston JS, Henson RNA, Fine-Goulden, MR, Dolan RJ (
Author notes
1Department of Cognitive Development and Laboratory of Neurophysiology, University of Louvain, Belgium, 2Department of Cognitive Neuroscience, Maastricht University, The Netherlands; F.C. Donders Centre for Cognitive Neuroimaging, Nijmegen, The Netherlands, 3Department of Psychology, University of Glasgow, UK and 4Department of Neurology, University Hospital Geneva, Switzerland


![Performance (response times) in between category and within-category object matching tasks. (a) P.S. responds normally fast in a between-category matching task for faces, birds, boats, cars and chairs. (b) P.S. responds abnormally slow in a within-category face matching task. (c) RTs of correct responses in a within-category matching task normalized by the corresponding RTs in a between-category matching task [(RTs within – RTs between)/(RTs within + RTs between)]. P.S. is significantly and selectively slower than control subjects in the within-category face matching condition.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cercor/16/4/10.1093/cercor/bhj005/2/m_cercorbhj005f02_ht.jpeg?Expires=1716358079&Signature=FV-~c6YeJhwOzhgsJZBAAARmqbol9inxrIQgds6VotNZ~Q7PlW56ATs4I6c79-isTDtrqMEzdPGnpcDup8R9qqwJu-9AzIvU~4d7WFJcWbkyo-poUPHMClRx0rQAdLpYdJFVe8dt1OXNFDmm-iyIdQfo~Wubs1KVIzgmwsgjFdekG4Tn53wtZDimMfbP2Yn-tFyc-jJueM3OwZHPgUW~gHVCJfC-As1R1DyukqgiLE~mc182q-toXjgWzaG-u3JwR2L8xJfdyyUplOwOPWdk7FCcJCTKKr16x5SoW5BNEuzQkmQHTMWszbkZknHvrSfJKovwWoIAQ5yvQruTRd85hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)