-
PDF
- Split View
-
Views
-
Cite
Cite
Khitam Muhsen, Myron M. Levine, A Systematic Review and Meta-analysis of the Association Between Giardia lamblia and Endemic Pediatric Diarrhea in Developing Countries, Clinical Infectious Diseases, Volume 55, Issue suppl_4, December 2012, Pages S271–S293, https://doi.org/10.1093/cid/cis762
Close - Share Icon Share
Abstract
We performed a systematic literature review and meta-analysis examining the association between diarrhea in young children in nonindustrialized settings and Giardia lamblia infection. Eligible were case/control and longitudinal studies that defined the outcome as acute or persistent (>14 days) diarrhea, adjusted for confounders and lasting for at least 1 year. Data on G. lamblia detection (mainly in stools) from diarrhea patients and controls without diarrhea were abstracted. Random effects model meta-analysis obtained pooled odds ratios (ORs) and 95% confidence intervals (CIs). Twelve nonindustrialized-setting acute pediatric diarrhea studies met the meta-analysis inclusion criteria. Random-effects model meta-analysis of combined results (9774 acute diarrhea cases and 8766 controls) yielded a pooled OR of 0.60 (95% CI, .38–.94; P = .03), indicating that G. lamblia was not associated with acute diarrhea. However, limited data suggest that initial Giardia infections in early infancy may be positively associated with diarrhea. Meta-analysis of 5 persistent diarrhea studies showed a pooled OR of 3.18 (95% CI, 1.50–6.76; P < .001), positively linking Giardia with that syndrome. The well-powered Global Enteric Multicenter Study (GEMS) is prospectively addressing the association between G. lamblia infection and diarrhea in children in developing countries.
Giardia lamblia (synonymous with Giardia duodenalis and Giardia intestinalis) is a unicellular eukaryotic microscopic enteric protozoa [1–4] that has been incriminated as a cause of diarrhea in individuals in both industrialized and developing countries [5–9]. When clinical illness ensues, it ranges from self-limited acute to persistent diarrhea [4, 10, 11], accompanied by malabsorption. The circumstances under which G. lamblia constitutes an etiologic agent of acute or persistent diarrheal disease are not well understood, since in other instances it colonizes without causing diarrhea and in yet other conditions it appears actually to protect against certain forms of diarrheal disease [12, 13].
Experimental challenge studies unequivocally document that some strains of G. lamblia can cause diarrhea in healthy adult volunteers [14, 15], and convincing epidemiological descriptions of acute gastroenteritis outbreaks also provide evidence that in certain hosts and settings this protozoan causes acute diarrhea [11, 16–23]. Finally, some case/control studies and longitudinal studies that prospectively follow cohorts of children (and occasionally adults) also support the notion that G. lamblia infection is associated with acute or persistent diarrhea [6, 7, 24, 25]. On the other hand, many other case/control and prospective cohort studies do not incriminate G. lamblia as a cause of diarrhea [26–28]; moreover, several studies suggest that carriage of this protozoan actually protects against diarrhea [12, 13, 29, 30].
Because of this confusing situation with respect to the role of G. lamblia as an enteric pathogen and the ensuing clinical and epidemiologic equipoise, we systematically reviewed the literature and performed a meta-analysis to examine the association between the occurrence of diarrheal disease in young children in developing countries and the presence of G. lamblia in their stool samples. We hypothesized that the association linking G. lamblia with diarrhea may differ whether one examines the clinical syndrome of acute vs persistent diarrhea; we further hypothesized that the association may be age-dependent. Finally, we reviewed the role of G. lamblia as a putative cause of traveler's diarrhea (albeit mainly in adults), anticipating that these data might shed additional light on the circumstances under which G. lamblia causes diarrhea.
METHODS
We performed a PubMed literature search (limited to English-language publications of human studies published prior to 1 April 2012) using the terms “Giardia and diarrhea,” “Giardia gastroenteritis,” “Giardia and travelers’ diarrhea,” and “etiology of travelers’ diarrhea.” To detect additional relevant publications, we used the PubMed option of “related articles” and checked the reference lists of the original and review articles.
Exclusion Criteria
Studies conducted in developed countries, case reports, case series, and studies conducted in patients with immunodeficiency or immunocompromise (eg, human immunodeficiency virus, cancer, post–organ transplantation) were excluded. Also excluded were cross-sectional studies and descriptive studies on the prevalence or detection proportion of G. lamblia in patients with diarrhea if they did not include a comparison/control group of subjects without diarrhea. If more than one report was published from the same study, only one report was included. The epidemiologic studies were critically reviewed with special emphasis on whether methodological limitations were evident.
Data Abstraction and Tabulation
Data on study design, study population, sampling frame and sample size, methods to detect G. lamblia, definition of diarrhea, case ascertainment, results, and matching or adjusting for potential confounders from each study were abstracted onto standardized forms.
Data extracted from the case/control studies included the number of diarrhea patients and control subjects without diarrhea and the number and/or proportion of cases and controls infected with G. lamblia. From the cohort studies we abstracted data on the number of stool samples that were obtained during diarrheal episodes, the number of stool samples that were obtained through routine surveillance in the absence of diarrhea, and the number and proportion of diarrheal and nondiarrheal stools that were positive for G. lamblia. Alternatively, depending on the design and the analysis in the original study, data were abstracted on the incidence of diarrheal disease in periods that were classified as G. lamblia positive or G. lamblia negative. Results stratified by age or other variables were abstracted if they were presented in the original articles. From studies that addressed the association between G. lamblia genotype and diarrhea, we abstracted data on the number of patients with diarrhea or gastrointestinal symptoms (cases), number of asymptomatic subjects (controls), the number and percentage of cases and controls infected with genotype A, and the number and percentage of the infected cases and controls with genotype B. If data on the rate ratio or odds ratio (OR) and 95% confidence intervals (CIs) or P value were presented in the original article, they were abstracted; otherwise we performed the calculations using WinPepi software version 11.15 [31].
Meta-analysis
Meta-analysis was performed to answer the question of whether G. lamblia infection is associated with an increased or a decreased risk of endemic diarrheal disease, using data that were generated by case/control or cohort studies. Pooled measurement of association was obtained using the random effects model and forest plots were generated to display summarized results. Heterogeneity among the studies was tested using heterogeneity χ2 test and I2 index [32]. Analyses were performed with stratification by the definition of the outcome (acute vs persistent diarrhea). Potential publication bias was assessed using funnel plots with the log OR of each study on the x-axis plotted against its standard error in the y-axis [33]. We also used the Egger regression intercept [34] to detect publication bias and we performed a cumulative meta-analysis (starting with the largest study) to assess the impact of the study size on the direction of the pooled risk estimate. The Comprehensive Meta-Analysis software package (version 2) was used to produce the analyses [35].
Meta-analysis was restricted to studies conducted in developing countries and other resource-poor settings that presented age-specific findings that allowed data abstraction of the results among children. Additional inclusion criteria were demonstration that matching or adjustment for potential confounders (eg, age, sex) was performed, the definition and duration of diarrhea were provided, and the study endured for at least 1 year (to account for seasonality). In the statistical analyses, we used the adjusted effect estimates of each study to obtain a pooled point estimate. However, if no multivariate analysis was conducted, we used the crude risk estimates.
A PRIMER ON G. LAMBLIA INFECTION
Because the biology of G. lamblia infection has been previously reviewed [2–4, 10, 36–40], only a few salient features are mentioned in this systematic review of the epidemiology.
The Life Cycle of G. lamblia
Giardia lamblia, a unicellular eukaryotic flagellated enteric protozoa [1–4] first described by van Leeuwenhoek in 1681 [2–4], occurs as a nonmotile cyst (responsible for transmission) or a motile trophozoite (associated with clinical symptoms) [4, 10]. Low gastric acidity followed by exposure to pancreatic secretions prompts excystation in the proximal small intestine, with 2 trophozoites deriving from each cyst. The trophozoites replicate in the lumen by binary fission and adhere to enterocytes of the proximal small intestine by suction (using their ventral adhesive disk) [1, 3, 4, 10] and by specific receptor-ligand interactions [36], but do not invade the epithelium. Encystation begins in the small intestine upon exposure to bile salts and is promoted by alkaline pH and decreasing cholesterol levels [1, 3, 4, 10, 41]. Both cysts and trophozoites may be excreted, depending on the nature of the stool. Giardia cysts survive in the environment for weeks and months [3, 4, 10], especially in cool and moist conditions [5].
Transmission
Giardia lamblia is transmitted via the ingestion of as few as 10 cysts [42]. Much information on the modes of transmission of Giardia comes from studies of infection and illness in industrialized-country settings. Waterborne transmission of G. lamblia is well documented [17, 20, 21, 43], including through recreational water activities and swimming [44–48]. The low inoculum facilitates person-to-person transmission among family members [18, 22, 49, 50] and subjects in crowded conditions where hygiene practices may be suboptimal (eg, daycare centers) [16, 18, 22]. Foodborne transmission of G. lamblia occurs but is uncommon [16, 51, 52]. Sexual transmission has been reported among men who have sex with men [53–56].
Epidemiological studies [57–61] and genotyping studies of G. lamblia support the possibility of zoonotic transmission [62, 63] of G. lamblia assemblages A and B, genotypes known to infect both humans and other host species; genotypes C to G infect only animals [64].
Giardia Clinical Illness
Analysis of responses of volunteers to ingestion of G. lamblia and descriptions of patients with disease consequent to well-described outbreaks attributed to the protozoan show that the main symptoms include diarrhea, abdominal pain, nausea, vomiting, flatulence, anorexia, and fever [4, 10, 11, 14]. In most instances the diarrheal illness is short-lived and self-limited. However, a proportion of individuals develop persistent diarrhea [4, 10, 11, 65], sometimes accompanied by malabsorption of sugars and fat and by weight loss. In both volunteers and outbreak situations, a sizable proportion of the infected subjects are asymptomatic, often exceeding the proportion who manifest clinical illness [65, 66].
THE ASSOCIATION BETWEEN G. LAMBLIA AND DIARRHEAL DISEASE
We identified 46 case/control studies and 18 longitudinal studies conducted from the 1970s through 2009 in developing-country and transitional populations that addressed the association between G. lamblia and endemic diarrheal disease.
Overview of the Case/Control Studies
Most of the case/control studies addressed the broad etiology or the role of protozoal agents in acute diarrheal disease, with G. lamblia being one of multiple enteropathogens looked for in stools [6, 7, 12, 13, 25, 26, 28, 67–103]. Children comprised the target population in the majority of the studies [6, 7, 12, 13, 25, 26, 28, 67, 68, 70–74, 76, 78, 79, 81, 82, 84–86, 88–91, 93–104], although some studies included adults with or without children [69, 75, 77, 80, 83, 87, 92, 105].
Case ascertainment was performed in the community [78, 96], outpatient clinics [6, 25, 28, 69, 73, 76, 81, 82, 91, 97, 102, 103], emergency rooms [83, 90, 95], or hospitals [7, 12, 13, 26, 68, 70–72, 75, 77, 80, 84, 86, 87, 93, 100, 104, 105]. The control subjects without diarrhea were outpatients [6, 13, 25, 28, 67, 69, 72, 73, 76, 80, 81, 89, 91, 95, 97, 101–103] emergency room patients [83], or hospitalized patients [7, 12, 13, 26, 70, 71, 75, 77, 84, 86, 87, 100, 104, 105], but some studies enrolled community controls [68, 78, 82, 93, 96]. Both inpatient and outpatient settings comprised the sampling frame for some studies [67, 74, 79, 85, 92, 98, 101].
Matching (or adjustment for confounders) between cases and controls by age, sex, and other variables was done in only a fraction of the studies [12, 13, 25, 28, 67, 68, 70, 72, 73, 75, 76, 78–80, 82, 83, 85, 86, 88, 90, 91, 93, 96–98, 102, 103, 105]. In others, neither matching nor adjustment for confounding effects in multivariate analyses was performed [6, 7, 26, 71, 74, 77, 81, 84, 87, 89, 92, 94, 95, 99–101, 104].
Some studies proceeded for at least 1 year [6, 7, 12, 13, 25, 28, 68–73, 75, 79, 80, 82, 84, 86, 90, 92–94, 96, 98, 100], while others lasted only a few months. Stool microscopy was the method most often used for detecting G. lamblia [6, 7, 12, 13, 25, 26, 28, 67–69, 71–79, 81–88, 90–97, 100–103]. In a few studies enzyme immunoassay [70, 80, 98, 105] or polymerase chain reaction [89] was used, either in addition to microscopy or as the exclusive method, to detect Giardia in stools or duodenal aspirates [104].
The outcome variable was “acute diarrhea” in the majority of the studies [26, 28, 68–73, 75, 76, 80–83, 85, 86, 88, 90, 92, 95, 98, 99, 101, 103, 105], but a few included “persistent diarrhea” as well as “acute diarrhea” [7, 25, 84, 97, 102, 104]; some studies presented pooled results of acute and persistent diarrhea. In 14 studies the length of the diarrheal episode was not specified [6, 12, 13, 67, 74, 77–79, 87, 89, 91, 93, 94, 96] or was not clearly defined [100]. A few studies focused on persistent diarrhea as the outcome variable [106–109], defined as diarrhea that continued unabated for >2 weeks; these studies are presented separately. In one study no operational definition was presented and in another study the authors reported on “chronic diarrhea,” defined as diarrhea that lasted >4 weeks [104].
Table 1 summarizes salient features of 12 pediatric case/control studies of acute diarrhea [28, 68, 70, 72, 73, 76, 80, 82, 86, 90, 98, 103] and 3 studies of persistent diarrhea [106, 107, 109] in which the authors controlled for potential confounders by matching or adjusting in multivariable analysis. From one study we abstracted data on children only [80].
Case/Control Studies on the Association Between Giardia lamblia and Diarrhea Among Children in Nonindustrialized Settingsa
| Study & Country . | Study Period . | Age . | Definition of Diarrhea . | Giardia Detection . | No. Cases Sampling Frame . | No. Controls Sampling Frame . | G. lamblia–Positive Cases, % . | G. lamblia–Positive Controls, % . | OR (95% CI) . | Matching/ Adjusting . |
|---|---|---|---|---|---|---|---|---|---|---|
| Acute Diarrhea | ||||||||||
| Orlandi [90] Brazil | 2000–02 | <6 y, 84.5% ≤2 y | Acute diarrhea: ≥3 loose stools in 24 h lasting ≥48 h | Microscopy (cysts) | 470 ER | 407 ER | 1.27% | 0.98% | 1.30 (.31–6.32) | Age, sex, SES |
| Huilan [82] Multicenter study in Mexico, Pakistan, China, Myanmar, India | 1982–85 | <3 y, 47%-75% <1 y | Acute diarrhea: an increase in the number or volume of stools that lasted for ≤72 h. Children with a history of blood or mucus in stools & a temperature of ≥38°C also included | Microscopy (trophozoites or cysts) | Total 3640 outpatient | 3279 community | 3% | 3% | 1.00 (.70–1.45) | Region, age, sex, SES, ethnicity |
| Chatterjee [72] India | 1982–83 | 0–14 y, 32.2% <1 y, 37.5% 1–4 y | Acute diarrhea | Microscopy (trophozoites or cysts) | 152 hospital | 272 health centers | 2.6% | Urban: 25.6% Rural: 15% | 0.10 (.04–.28) 0.15 (.04–.49) | Age |
| Mubashir [86] Pakistan | 1983–85 | <3 y, 73.6% 1–12 mo | Acute diarrhea of <72 h | Microscopy | 402 hospital | 402 hospital | 2% | 8.2% | 0.23 (.10–.48) | Age, sex, SES, geographic region, ethnicity |
| Albert [68] Bangladesh | 1994 | 0–5 y, 80% ≤2 y | Acute diarrhea ≥3 stools/day | Microscopy | 814 ICDDR,B | 814 community | 0.8% | 2.9% | 0.30 (.12–.68) | Age, neighborhood |
| Haque [80] Bangladeshb | 2004–06 | All ages: cases 30% 0–12 mo, controls 19% 0–12 mo | Acute diarrhea: ≥3 abnormal stools in 24 h. Dysentery: the presence of red blood cells, macrophages, or pus cells | EIA | 1760 ICDDR,B | 1145 clinic | 4.5% | 15.6% | 0.26 (.19–.34) | Age, sex, SES |
| Hoge [103] Nepal | 1994 | 0.5–5 y, mean age cases 19 mo | Acute diarrhea >3 unformed stools/24 h | Microscopy | 124 outpatient | 103 community | 13% | 18% | 0.65 (.31–1.36) | Age, sex, neighborhood |
| Echeverria [73] Thailand | 1985–86 | <5 y, 80% <2 y | Acute diarrhea: ≥3 loose stools in the previous 24 h for <72 h | Microscopy | 1230 outpatient | 1230 outpatient | 2% | 1.3% | 1.57 (.84–3.02) | Age |
| Bodhidatta [70] Thailand | 2001–02 | 3 mo to 5 y, 75% <2 y | Admission due to acute diarrhea | EIA | 207 hospital | 227 hospital | 15% | 23% | 0.58 (.35–.94) | Age |
| Loening [28] South Africa | 1985–86 | <6 y, 83% ≤2 y | ≥5 stools/day for >1 d & <7 d | Microscopy (trophozoites or cysts) | 373 outpatient | 371 outpatient | 6.4% | 5.9% | 1.09 (.60–2.00) | Age, clinic |
| Gascon [76] Tanzaniac | 1997 | 0–5 y, mean age: cases 1.9 y, controls 1.6 y | Acute diarrhea ≥3 watery/loose stools/24 h | Microscopy (trophozoites or cysts) Trophozoites | 103 clinic | 206 clinic | 14.5% | 15.5% | 1.06 (.51–2.19) 1.82 (.76–4.34) | Age, sex, no. of alive siblings, distance to water source, & having a latrine at home |
| Meng [98] Cambodiac | 2004–06 | 3 mo to 5 y, mean age: cases 11.4 mo, controls 31.2 mo | Acute diarrhea ≥3 watery/loose stools/24 h with ≥1 other enteric symptom | EIA | 569 inpatient & outpatient | 568 inpatient & outpatient | 8.3% | 21.7% | 0.63 (.40–.99) | Age, sex, season |
| Persistent Diarrhea | ||||||||||
| Sullivan [107] Gambiad | NA | 0.5–3 y | >3 loose stools/day persisting for >2 wk | Microscopy | 31 outpatient | 33 healthy children outpatient | 45% | 12% | 5.97 (1.50–28.20) | Age, sex |
| Bhandari [106] Indiae | NA | 0–36 mo | Persistent diarrhea ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 175 household surveillance | 175 healthy children; 175 acute diarrhea patients | 20% | 4.6% in each group | 5.22 (2.40–12.32) | Age, nutritional status |
| Mukhopadhyay [109] Nepalf | 1998–2004 | <5 y | Persistent diarrhea: ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 253 inpatient, outpatient | 100 healthy community controls, 100 acute diarrhea controls | Trophozoites: 9.8% Cysts: 14.2% | Trophozoites: healthy controls 2%; acute diarrhea 0% Cysts: healthy controls 6%; acute diarrhea 4% | Trophozoites: 5.37 (1.29–47.5) Cysts: 2.60 (1.03–7.79) | Nutritional status |
| Study & Country . | Study Period . | Age . | Definition of Diarrhea . | Giardia Detection . | No. Cases Sampling Frame . | No. Controls Sampling Frame . | G. lamblia–Positive Cases, % . | G. lamblia–Positive Controls, % . | OR (95% CI) . | Matching/ Adjusting . |
|---|---|---|---|---|---|---|---|---|---|---|
| Acute Diarrhea | ||||||||||
| Orlandi [90] Brazil | 2000–02 | <6 y, 84.5% ≤2 y | Acute diarrhea: ≥3 loose stools in 24 h lasting ≥48 h | Microscopy (cysts) | 470 ER | 407 ER | 1.27% | 0.98% | 1.30 (.31–6.32) | Age, sex, SES |
| Huilan [82] Multicenter study in Mexico, Pakistan, China, Myanmar, India | 1982–85 | <3 y, 47%-75% <1 y | Acute diarrhea: an increase in the number or volume of stools that lasted for ≤72 h. Children with a history of blood or mucus in stools & a temperature of ≥38°C also included | Microscopy (trophozoites or cysts) | Total 3640 outpatient | 3279 community | 3% | 3% | 1.00 (.70–1.45) | Region, age, sex, SES, ethnicity |
| Chatterjee [72] India | 1982–83 | 0–14 y, 32.2% <1 y, 37.5% 1–4 y | Acute diarrhea | Microscopy (trophozoites or cysts) | 152 hospital | 272 health centers | 2.6% | Urban: 25.6% Rural: 15% | 0.10 (.04–.28) 0.15 (.04–.49) | Age |
| Mubashir [86] Pakistan | 1983–85 | <3 y, 73.6% 1–12 mo | Acute diarrhea of <72 h | Microscopy | 402 hospital | 402 hospital | 2% | 8.2% | 0.23 (.10–.48) | Age, sex, SES, geographic region, ethnicity |
| Albert [68] Bangladesh | 1994 | 0–5 y, 80% ≤2 y | Acute diarrhea ≥3 stools/day | Microscopy | 814 ICDDR,B | 814 community | 0.8% | 2.9% | 0.30 (.12–.68) | Age, neighborhood |
| Haque [80] Bangladeshb | 2004–06 | All ages: cases 30% 0–12 mo, controls 19% 0–12 mo | Acute diarrhea: ≥3 abnormal stools in 24 h. Dysentery: the presence of red blood cells, macrophages, or pus cells | EIA | 1760 ICDDR,B | 1145 clinic | 4.5% | 15.6% | 0.26 (.19–.34) | Age, sex, SES |
| Hoge [103] Nepal | 1994 | 0.5–5 y, mean age cases 19 mo | Acute diarrhea >3 unformed stools/24 h | Microscopy | 124 outpatient | 103 community | 13% | 18% | 0.65 (.31–1.36) | Age, sex, neighborhood |
| Echeverria [73] Thailand | 1985–86 | <5 y, 80% <2 y | Acute diarrhea: ≥3 loose stools in the previous 24 h for <72 h | Microscopy | 1230 outpatient | 1230 outpatient | 2% | 1.3% | 1.57 (.84–3.02) | Age |
| Bodhidatta [70] Thailand | 2001–02 | 3 mo to 5 y, 75% <2 y | Admission due to acute diarrhea | EIA | 207 hospital | 227 hospital | 15% | 23% | 0.58 (.35–.94) | Age |
| Loening [28] South Africa | 1985–86 | <6 y, 83% ≤2 y | ≥5 stools/day for >1 d & <7 d | Microscopy (trophozoites or cysts) | 373 outpatient | 371 outpatient | 6.4% | 5.9% | 1.09 (.60–2.00) | Age, clinic |
| Gascon [76] Tanzaniac | 1997 | 0–5 y, mean age: cases 1.9 y, controls 1.6 y | Acute diarrhea ≥3 watery/loose stools/24 h | Microscopy (trophozoites or cysts) Trophozoites | 103 clinic | 206 clinic | 14.5% | 15.5% | 1.06 (.51–2.19) 1.82 (.76–4.34) | Age, sex, no. of alive siblings, distance to water source, & having a latrine at home |
| Meng [98] Cambodiac | 2004–06 | 3 mo to 5 y, mean age: cases 11.4 mo, controls 31.2 mo | Acute diarrhea ≥3 watery/loose stools/24 h with ≥1 other enteric symptom | EIA | 569 inpatient & outpatient | 568 inpatient & outpatient | 8.3% | 21.7% | 0.63 (.40–.99) | Age, sex, season |
| Persistent Diarrhea | ||||||||||
| Sullivan [107] Gambiad | NA | 0.5–3 y | >3 loose stools/day persisting for >2 wk | Microscopy | 31 outpatient | 33 healthy children outpatient | 45% | 12% | 5.97 (1.50–28.20) | Age, sex |
| Bhandari [106] Indiae | NA | 0–36 mo | Persistent diarrhea ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 175 household surveillance | 175 healthy children; 175 acute diarrhea patients | 20% | 4.6% in each group | 5.22 (2.40–12.32) | Age, nutritional status |
| Mukhopadhyay [109] Nepalf | 1998–2004 | <5 y | Persistent diarrhea: ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 253 inpatient, outpatient | 100 healthy community controls, 100 acute diarrhea controls | Trophozoites: 9.8% Cysts: 14.2% | Trophozoites: healthy controls 2%; acute diarrhea 0% Cysts: healthy controls 6%; acute diarrhea 4% | Trophozoites: 5.37 (1.29–47.5) Cysts: 2.60 (1.03–7.79) | Nutritional status |
Abbreviations: CI, confidence interval; EIA, enzyme immunoassay; ER, emergency room; ICDDR,B, International Centre for Diarrhoeal Disease Research, Bangladesh; OR, odds ratio; SES, socioeconomic status.
a The ORs and 95% CIs were calculated using the raw data that were presented in the original manuscripts, except for 2 studies that presented adjusted OR: Gascon et al [76] and Meng et al [98].
b From the study of Haque et al [80] we abstracted data only on children ≤5 years of age.
c The adjusted ORs that appeared in the manuscript are presented.
d Cases were children with chronic diarrhea and malnutrition; data on the healthy control children are presented.
e The results are similar when the control group was the healthy children or the patients with acute diarrhea.
f OR was calculated while including the healthy control children.
Case/Control Studies on the Association Between Giardia lamblia and Diarrhea Among Children in Nonindustrialized Settingsa
| Study & Country . | Study Period . | Age . | Definition of Diarrhea . | Giardia Detection . | No. Cases Sampling Frame . | No. Controls Sampling Frame . | G. lamblia–Positive Cases, % . | G. lamblia–Positive Controls, % . | OR (95% CI) . | Matching/ Adjusting . |
|---|---|---|---|---|---|---|---|---|---|---|
| Acute Diarrhea | ||||||||||
| Orlandi [90] Brazil | 2000–02 | <6 y, 84.5% ≤2 y | Acute diarrhea: ≥3 loose stools in 24 h lasting ≥48 h | Microscopy (cysts) | 470 ER | 407 ER | 1.27% | 0.98% | 1.30 (.31–6.32) | Age, sex, SES |
| Huilan [82] Multicenter study in Mexico, Pakistan, China, Myanmar, India | 1982–85 | <3 y, 47%-75% <1 y | Acute diarrhea: an increase in the number or volume of stools that lasted for ≤72 h. Children with a history of blood or mucus in stools & a temperature of ≥38°C also included | Microscopy (trophozoites or cysts) | Total 3640 outpatient | 3279 community | 3% | 3% | 1.00 (.70–1.45) | Region, age, sex, SES, ethnicity |
| Chatterjee [72] India | 1982–83 | 0–14 y, 32.2% <1 y, 37.5% 1–4 y | Acute diarrhea | Microscopy (trophozoites or cysts) | 152 hospital | 272 health centers | 2.6% | Urban: 25.6% Rural: 15% | 0.10 (.04–.28) 0.15 (.04–.49) | Age |
| Mubashir [86] Pakistan | 1983–85 | <3 y, 73.6% 1–12 mo | Acute diarrhea of <72 h | Microscopy | 402 hospital | 402 hospital | 2% | 8.2% | 0.23 (.10–.48) | Age, sex, SES, geographic region, ethnicity |
| Albert [68] Bangladesh | 1994 | 0–5 y, 80% ≤2 y | Acute diarrhea ≥3 stools/day | Microscopy | 814 ICDDR,B | 814 community | 0.8% | 2.9% | 0.30 (.12–.68) | Age, neighborhood |
| Haque [80] Bangladeshb | 2004–06 | All ages: cases 30% 0–12 mo, controls 19% 0–12 mo | Acute diarrhea: ≥3 abnormal stools in 24 h. Dysentery: the presence of red blood cells, macrophages, or pus cells | EIA | 1760 ICDDR,B | 1145 clinic | 4.5% | 15.6% | 0.26 (.19–.34) | Age, sex, SES |
| Hoge [103] Nepal | 1994 | 0.5–5 y, mean age cases 19 mo | Acute diarrhea >3 unformed stools/24 h | Microscopy | 124 outpatient | 103 community | 13% | 18% | 0.65 (.31–1.36) | Age, sex, neighborhood |
| Echeverria [73] Thailand | 1985–86 | <5 y, 80% <2 y | Acute diarrhea: ≥3 loose stools in the previous 24 h for <72 h | Microscopy | 1230 outpatient | 1230 outpatient | 2% | 1.3% | 1.57 (.84–3.02) | Age |
| Bodhidatta [70] Thailand | 2001–02 | 3 mo to 5 y, 75% <2 y | Admission due to acute diarrhea | EIA | 207 hospital | 227 hospital | 15% | 23% | 0.58 (.35–.94) | Age |
| Loening [28] South Africa | 1985–86 | <6 y, 83% ≤2 y | ≥5 stools/day for >1 d & <7 d | Microscopy (trophozoites or cysts) | 373 outpatient | 371 outpatient | 6.4% | 5.9% | 1.09 (.60–2.00) | Age, clinic |
| Gascon [76] Tanzaniac | 1997 | 0–5 y, mean age: cases 1.9 y, controls 1.6 y | Acute diarrhea ≥3 watery/loose stools/24 h | Microscopy (trophozoites or cysts) Trophozoites | 103 clinic | 206 clinic | 14.5% | 15.5% | 1.06 (.51–2.19) 1.82 (.76–4.34) | Age, sex, no. of alive siblings, distance to water source, & having a latrine at home |
| Meng [98] Cambodiac | 2004–06 | 3 mo to 5 y, mean age: cases 11.4 mo, controls 31.2 mo | Acute diarrhea ≥3 watery/loose stools/24 h with ≥1 other enteric symptom | EIA | 569 inpatient & outpatient | 568 inpatient & outpatient | 8.3% | 21.7% | 0.63 (.40–.99) | Age, sex, season |
| Persistent Diarrhea | ||||||||||
| Sullivan [107] Gambiad | NA | 0.5–3 y | >3 loose stools/day persisting for >2 wk | Microscopy | 31 outpatient | 33 healthy children outpatient | 45% | 12% | 5.97 (1.50–28.20) | Age, sex |
| Bhandari [106] Indiae | NA | 0–36 mo | Persistent diarrhea ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 175 household surveillance | 175 healthy children; 175 acute diarrhea patients | 20% | 4.6% in each group | 5.22 (2.40–12.32) | Age, nutritional status |
| Mukhopadhyay [109] Nepalf | 1998–2004 | <5 y | Persistent diarrhea: ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 253 inpatient, outpatient | 100 healthy community controls, 100 acute diarrhea controls | Trophozoites: 9.8% Cysts: 14.2% | Trophozoites: healthy controls 2%; acute diarrhea 0% Cysts: healthy controls 6%; acute diarrhea 4% | Trophozoites: 5.37 (1.29–47.5) Cysts: 2.60 (1.03–7.79) | Nutritional status |
| Study & Country . | Study Period . | Age . | Definition of Diarrhea . | Giardia Detection . | No. Cases Sampling Frame . | No. Controls Sampling Frame . | G. lamblia–Positive Cases, % . | G. lamblia–Positive Controls, % . | OR (95% CI) . | Matching/ Adjusting . |
|---|---|---|---|---|---|---|---|---|---|---|
| Acute Diarrhea | ||||||||||
| Orlandi [90] Brazil | 2000–02 | <6 y, 84.5% ≤2 y | Acute diarrhea: ≥3 loose stools in 24 h lasting ≥48 h | Microscopy (cysts) | 470 ER | 407 ER | 1.27% | 0.98% | 1.30 (.31–6.32) | Age, sex, SES |
| Huilan [82] Multicenter study in Mexico, Pakistan, China, Myanmar, India | 1982–85 | <3 y, 47%-75% <1 y | Acute diarrhea: an increase in the number or volume of stools that lasted for ≤72 h. Children with a history of blood or mucus in stools & a temperature of ≥38°C also included | Microscopy (trophozoites or cysts) | Total 3640 outpatient | 3279 community | 3% | 3% | 1.00 (.70–1.45) | Region, age, sex, SES, ethnicity |
| Chatterjee [72] India | 1982–83 | 0–14 y, 32.2% <1 y, 37.5% 1–4 y | Acute diarrhea | Microscopy (trophozoites or cysts) | 152 hospital | 272 health centers | 2.6% | Urban: 25.6% Rural: 15% | 0.10 (.04–.28) 0.15 (.04–.49) | Age |
| Mubashir [86] Pakistan | 1983–85 | <3 y, 73.6% 1–12 mo | Acute diarrhea of <72 h | Microscopy | 402 hospital | 402 hospital | 2% | 8.2% | 0.23 (.10–.48) | Age, sex, SES, geographic region, ethnicity |
| Albert [68] Bangladesh | 1994 | 0–5 y, 80% ≤2 y | Acute diarrhea ≥3 stools/day | Microscopy | 814 ICDDR,B | 814 community | 0.8% | 2.9% | 0.30 (.12–.68) | Age, neighborhood |
| Haque [80] Bangladeshb | 2004–06 | All ages: cases 30% 0–12 mo, controls 19% 0–12 mo | Acute diarrhea: ≥3 abnormal stools in 24 h. Dysentery: the presence of red blood cells, macrophages, or pus cells | EIA | 1760 ICDDR,B | 1145 clinic | 4.5% | 15.6% | 0.26 (.19–.34) | Age, sex, SES |
| Hoge [103] Nepal | 1994 | 0.5–5 y, mean age cases 19 mo | Acute diarrhea >3 unformed stools/24 h | Microscopy | 124 outpatient | 103 community | 13% | 18% | 0.65 (.31–1.36) | Age, sex, neighborhood |
| Echeverria [73] Thailand | 1985–86 | <5 y, 80% <2 y | Acute diarrhea: ≥3 loose stools in the previous 24 h for <72 h | Microscopy | 1230 outpatient | 1230 outpatient | 2% | 1.3% | 1.57 (.84–3.02) | Age |
| Bodhidatta [70] Thailand | 2001–02 | 3 mo to 5 y, 75% <2 y | Admission due to acute diarrhea | EIA | 207 hospital | 227 hospital | 15% | 23% | 0.58 (.35–.94) | Age |
| Loening [28] South Africa | 1985–86 | <6 y, 83% ≤2 y | ≥5 stools/day for >1 d & <7 d | Microscopy (trophozoites or cysts) | 373 outpatient | 371 outpatient | 6.4% | 5.9% | 1.09 (.60–2.00) | Age, clinic |
| Gascon [76] Tanzaniac | 1997 | 0–5 y, mean age: cases 1.9 y, controls 1.6 y | Acute diarrhea ≥3 watery/loose stools/24 h | Microscopy (trophozoites or cysts) Trophozoites | 103 clinic | 206 clinic | 14.5% | 15.5% | 1.06 (.51–2.19) 1.82 (.76–4.34) | Age, sex, no. of alive siblings, distance to water source, & having a latrine at home |
| Meng [98] Cambodiac | 2004–06 | 3 mo to 5 y, mean age: cases 11.4 mo, controls 31.2 mo | Acute diarrhea ≥3 watery/loose stools/24 h with ≥1 other enteric symptom | EIA | 569 inpatient & outpatient | 568 inpatient & outpatient | 8.3% | 21.7% | 0.63 (.40–.99) | Age, sex, season |
| Persistent Diarrhea | ||||||||||
| Sullivan [107] Gambiad | NA | 0.5–3 y | >3 loose stools/day persisting for >2 wk | Microscopy | 31 outpatient | 33 healthy children outpatient | 45% | 12% | 5.97 (1.50–28.20) | Age, sex |
| Bhandari [106] Indiae | NA | 0–36 mo | Persistent diarrhea ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 175 household surveillance | 175 healthy children; 175 acute diarrhea patients | 20% | 4.6% in each group | 5.22 (2.40–12.32) | Age, nutritional status |
| Mukhopadhyay [109] Nepalf | 1998–2004 | <5 y | Persistent diarrhea: ≥3 liquid stools in 24 h lasting ≥14 d; acute diarrhea (<14 d). | Microscopy | 253 inpatient, outpatient | 100 healthy community controls, 100 acute diarrhea controls | Trophozoites: 9.8% Cysts: 14.2% | Trophozoites: healthy controls 2%; acute diarrhea 0% Cysts: healthy controls 6%; acute diarrhea 4% | Trophozoites: 5.37 (1.29–47.5) Cysts: 2.60 (1.03–7.79) | Nutritional status |
Abbreviations: CI, confidence interval; EIA, enzyme immunoassay; ER, emergency room; ICDDR,B, International Centre for Diarrhoeal Disease Research, Bangladesh; OR, odds ratio; SES, socioeconomic status.
a The ORs and 95% CIs were calculated using the raw data that were presented in the original manuscripts, except for 2 studies that presented adjusted OR: Gascon et al [76] and Meng et al [98].
b From the study of Haque et al [80] we abstracted data only on children ≤5 years of age.
c The adjusted ORs that appeared in the manuscript are presented.
d Cases were children with chronic diarrhea and malnutrition; data on the healthy control children are presented.
e The results are similar when the control group was the healthy children or the patients with acute diarrhea.
f OR was calculated while including the healthy control children.
Acute Diarrhea
Among studies conducted in children in developing countries or other nonindustrialized settings, 6 studies showed no significant difference between cases and controls in the detection rate of G. lamblia [28, 73, 76, 82, 90, 103], whereas 6 other studies showed a significantly lower detection rate of G. lamblia in stools from patients with acute diarrhea than from controls [68, 70, 72, 80, 86, 98].
Persistent Diarrhea
Five case/control studies examined the association between G. lamblia and persistent diarrhea (≥14 days duration); 4 studies were carried out among children [104, 106, 107, 109] and one study enrolled adults [108]. Table 1 presents the pediatric studies that matched cases and controls according to potential confounders. The detection rate of G. lamblia was high in subjects with persistent diarrhea (9.8%–45%) and was 2.6- to 5.9-fold higher than in the control group [106, 107, 109].
Overview of the Longitudinal Studies
The salient features of the study designs and the results from 18 longitudinal studies undertaken in developing countries [24, 27, 30, 110–121] or populations in transition [29, 122, 123] are presented in Table 2. Some longitudinal cohort studies addressed the epidemiology and broad etiology of diarrheal disease [24, 110, 112, 113, 115–121], whereas others confined themselves to addressing the etiologic role of G. lamblia in association with diarrhea [27, 29, 30, 111, 114, 122, 123]. Children comprised the study target population except for 2 studies, one from Brazil [110] and the other from Egypt [112], that also included adult household members. The follow-up period in most studies was approximately 24 months [24, 29, 110, 112, 113, 116, 118, 120, 122, 123]. In the remaining studies follow-up was approximately 10–12 months [30, 114, 115, 117, 119], 3 years (average 1.5 years) [111], or 4 years (median 23 months) [27].
Cohort Studies That Addressed the Role of Giardia lamblia in Diarrhea
| Study & Country . | Study Population . | Definition of Diarrhea . | Surveillance of Diarrhea . | Detection of Giardia . | No. Diarrheal Stools . | No. Nondiarrheal Stools . | G. lamblia– Positive Diarrhea Stools, % . | G. lamblia– Positive Nondiarrhea Stools, % . | OR/RR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|
| Guerrant [110] Brazil | 297, household members, all ages | A significant change in bowel habits: decreased consistency or increased frequency. The duration of diarrhea was presented for other pathogens than Giardia. | Daily surveillance was conducted by the mother, and by weekly home visits performed by research assistants. | Microscopy | 150 | 32 | 6.7% | 12.5% | 0.50 (.13–2.35) |
| Schorling [113] Brazilb | 175, age <5 y | An increase in stool frequency or decrease in consistency, which lasted ≥1 d and was separated from another episode by 3 diarrhea-free days. Acute diarrhea <14 d. Persistent diarrhea ≥14 d, | Home visits 3 times/wk | Microscopy | Acute diarrhea: 50, persistent diarrhea, 40 | 38 | Acute diarrhea: 22%, persistent diarrhea:17.5% | 13.2% | 1.86 (.59–6.45) 1.40 (.39–5.26) |
| Newman [111] Brazil | 157 newborns followed up from birth | ≥3 unformed stools in 24 h. Acute diarrhea lasting <14 d. Persistent diarrhea ≥14 d. | Home visits 3 times/wk. | Microscopy | Acute diarrhea: 514, persistent diarrhea: 97 | 299 | Acute diarrhea: 7.6%, persistent diarrhea: 20.6% | 7.4% | 1.03 (.58–1.84) 3.27 (1.62–6.62) |
| Black [116] Peru | 153 newborns followed from birth | ≥1 d with liquid stools totaling 6 for infants <1 mo, 5 for infants aged 1 mo & 4 for older infants. New episode began after 2 free-illness days. The duration of diarrhea was presented for other pathogens than Giardia | Thrice-weekly home visits | Microscopy | 952 | 1973 | 0.7% | 0.8% | 0.91 (.35–2.18) |
| Kaminsky [119] Honduras | 266, 101 controls, Age <6 y | An increase in the usual number & change in the consistency of stools for ≥1 d. Acute & persistent diarrhea | Twice-weekly visits | Microscopy (troph. or cysts) | 848 | 101 | 29% | 57% | 0.50 (.42–.63) |
| Hollm-Delgado [27] Peruc | 220 infants followed up from birth to age 35 mo | ≥3 liquid/semi liquid stools/d in 2 consecutive days. The duration of diarrhea was not presented. | Daily home visits | Microscopy | 3911 | 16 973 | 6% | 6.2% | 0.95 (.79–1.13) |
| Boeke [121] Colombiad | 442, age 5–12 y | Maternal reports. The outcome was diarrhea days. | Daily reports in pictorial diaries | Microscopy (cysts) | Positive children: 28 | Negative children: 414 | 4.0 | 4.7 | 0.73 (.52–1.02) |
| Stanton [24] Bangladesh | 343, age <6 y | ≥ 3 loose stools in 24 h; new episode began after 14 d without diarrhea. Duration of diarrhea was not presented. | Fortnightly maternal interviews | Microscopy (cysts) | 225 | 1006 | 11% | 4% | 2.61 (1.53–4.37) |
| Baqui [117] Bangladesh | 705, age <5 y | ≥3 liquid/loose or watery stools or at least 1 bloody stool in 24-hours, Acute diarrhea <14 d, persistent diarrhea ≥4 d | Home visits every fourth day. | Microscopy | Acute diarrhea: 161 Persistent diarrhea: 167 | 165 | Acute diarrhea: 0.6% Persistent diarrhea: 1.2% | 1.8% | 0.33 0.68 |
| Hasan [118] Bangladesh | 252 newborns followed from birth for 2 y | ≥3 liquid stools in 24 h or any loose stools accompanied with blood in 24 h. Acute diarrhea <2 wk. Persistent diarrhea ≥2 wk. Data for Giardia were presented in a pooled analysis of acute & persistent diarrhea. | Twice-weekly home visits | Microscopy (troph.) | 1748 | 5679 | 13.2% | 13% | 1.01 (.86–1.19) |
| Zaki [112] Egypt | 2563, household members, all ages | Reports of the family speak person. The duration of diarrhea was not presented | Twice-weekly home visits | Microscopy | 3080 | 703 | 44.3% | 56.0% | 0.63 (.53–.74) |
| Fraser [123] Israel | 164 Bedouin newborns followed from birth to age 23 mo | ≥3 soft stools in 24 hours. For infants aged <1 mo ≥4 soft stools. The duration of diarrhea was not presented. | Through the local clinics and hospital, and through monthly and weekly maternal interviews. | Microscopy (cysts) | 239 | 730 | 22.3% | 28.5% | 0.8 (.7–.9) |
| Bilenko [29] Israele | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 349 | 8591 | 16% | 23% | 0.65 (.47–.91) |
| Bilenko [29] Israelf | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 1453 Giardia-positive months | 3001 Giardia-negative months | 6.7% | 6.7% | 1.09 (.81–1.46) |
| Molbak [115] Guinea-Bissau | 471–755 children | Maternal reports. Data for Giardia were presented in a pooled analysis of acute and persistent diarrhea. | Weekly visits | Microscopy (troph. or cysts) Troph. | 1219 | 511 | 19.1%, 9.3% | 25%, 9.8% | 0.8 (.6–1.0) 1.1 (.7–1.5) |
| Chunge [114] Kenyag | 84 children aged 10–28 mo | Maternal report. The duration of diarrhea was not presented. | Weekly surveillance | Microscopy (troph. or cysts) | 1227 | 537 | 78.8% | 68.6% | 1.69 (1.15–2.54) |
| Veenemans [30] Tanzaniah | 558, age 6–60 mo | Diarrhea: any report by the caretaker or ≥3 stools in 24 h. The duration of diarrhea was not presented. | Health-facility based surveillance | EIA | Positive children: 192 | Negative children: 336 | Overall: 0.43 Micro nutrients: 0.58 No micro nutrients: 0.29 | 0.68 0.63 0.72 | 0.84 (.64–1.09) 1.04 (.75–1.43) 0.56 (.34–.90) |
| Valentiner-Branth [120] Guinea-Bissaui | 200 newborns followed from birth to age 2 y | Maternal report. The duration of episode was not presented. | Weekly home visits | Microscopy | na | na | na | na | 0.64 (.46–.89) |
| Study & Country . | Study Population . | Definition of Diarrhea . | Surveillance of Diarrhea . | Detection of Giardia . | No. Diarrheal Stools . | No. Nondiarrheal Stools . | G. lamblia– Positive Diarrhea Stools, % . | G. lamblia– Positive Nondiarrhea Stools, % . | OR/RR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|
| Guerrant [110] Brazil | 297, household members, all ages | A significant change in bowel habits: decreased consistency or increased frequency. The duration of diarrhea was presented for other pathogens than Giardia. | Daily surveillance was conducted by the mother, and by weekly home visits performed by research assistants. | Microscopy | 150 | 32 | 6.7% | 12.5% | 0.50 (.13–2.35) |
| Schorling [113] Brazilb | 175, age <5 y | An increase in stool frequency or decrease in consistency, which lasted ≥1 d and was separated from another episode by 3 diarrhea-free days. Acute diarrhea <14 d. Persistent diarrhea ≥14 d, | Home visits 3 times/wk | Microscopy | Acute diarrhea: 50, persistent diarrhea, 40 | 38 | Acute diarrhea: 22%, persistent diarrhea:17.5% | 13.2% | 1.86 (.59–6.45) 1.40 (.39–5.26) |
| Newman [111] Brazil | 157 newborns followed up from birth | ≥3 unformed stools in 24 h. Acute diarrhea lasting <14 d. Persistent diarrhea ≥14 d. | Home visits 3 times/wk. | Microscopy | Acute diarrhea: 514, persistent diarrhea: 97 | 299 | Acute diarrhea: 7.6%, persistent diarrhea: 20.6% | 7.4% | 1.03 (.58–1.84) 3.27 (1.62–6.62) |
| Black [116] Peru | 153 newborns followed from birth | ≥1 d with liquid stools totaling 6 for infants <1 mo, 5 for infants aged 1 mo & 4 for older infants. New episode began after 2 free-illness days. The duration of diarrhea was presented for other pathogens than Giardia | Thrice-weekly home visits | Microscopy | 952 | 1973 | 0.7% | 0.8% | 0.91 (.35–2.18) |
| Kaminsky [119] Honduras | 266, 101 controls, Age <6 y | An increase in the usual number & change in the consistency of stools for ≥1 d. Acute & persistent diarrhea | Twice-weekly visits | Microscopy (troph. or cysts) | 848 | 101 | 29% | 57% | 0.50 (.42–.63) |
| Hollm-Delgado [27] Peruc | 220 infants followed up from birth to age 35 mo | ≥3 liquid/semi liquid stools/d in 2 consecutive days. The duration of diarrhea was not presented. | Daily home visits | Microscopy | 3911 | 16 973 | 6% | 6.2% | 0.95 (.79–1.13) |
| Boeke [121] Colombiad | 442, age 5–12 y | Maternal reports. The outcome was diarrhea days. | Daily reports in pictorial diaries | Microscopy (cysts) | Positive children: 28 | Negative children: 414 | 4.0 | 4.7 | 0.73 (.52–1.02) |
| Stanton [24] Bangladesh | 343, age <6 y | ≥ 3 loose stools in 24 h; new episode began after 14 d without diarrhea. Duration of diarrhea was not presented. | Fortnightly maternal interviews | Microscopy (cysts) | 225 | 1006 | 11% | 4% | 2.61 (1.53–4.37) |
| Baqui [117] Bangladesh | 705, age <5 y | ≥3 liquid/loose or watery stools or at least 1 bloody stool in 24-hours, Acute diarrhea <14 d, persistent diarrhea ≥4 d | Home visits every fourth day. | Microscopy | Acute diarrhea: 161 Persistent diarrhea: 167 | 165 | Acute diarrhea: 0.6% Persistent diarrhea: 1.2% | 1.8% | 0.33 0.68 |
| Hasan [118] Bangladesh | 252 newborns followed from birth for 2 y | ≥3 liquid stools in 24 h or any loose stools accompanied with blood in 24 h. Acute diarrhea <2 wk. Persistent diarrhea ≥2 wk. Data for Giardia were presented in a pooled analysis of acute & persistent diarrhea. | Twice-weekly home visits | Microscopy (troph.) | 1748 | 5679 | 13.2% | 13% | 1.01 (.86–1.19) |
| Zaki [112] Egypt | 2563, household members, all ages | Reports of the family speak person. The duration of diarrhea was not presented | Twice-weekly home visits | Microscopy | 3080 | 703 | 44.3% | 56.0% | 0.63 (.53–.74) |
| Fraser [123] Israel | 164 Bedouin newborns followed from birth to age 23 mo | ≥3 soft stools in 24 hours. For infants aged <1 mo ≥4 soft stools. The duration of diarrhea was not presented. | Through the local clinics and hospital, and through monthly and weekly maternal interviews. | Microscopy (cysts) | 239 | 730 | 22.3% | 28.5% | 0.8 (.7–.9) |
| Bilenko [29] Israele | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 349 | 8591 | 16% | 23% | 0.65 (.47–.91) |
| Bilenko [29] Israelf | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 1453 Giardia-positive months | 3001 Giardia-negative months | 6.7% | 6.7% | 1.09 (.81–1.46) |
| Molbak [115] Guinea-Bissau | 471–755 children | Maternal reports. Data for Giardia were presented in a pooled analysis of acute and persistent diarrhea. | Weekly visits | Microscopy (troph. or cysts) Troph. | 1219 | 511 | 19.1%, 9.3% | 25%, 9.8% | 0.8 (.6–1.0) 1.1 (.7–1.5) |
| Chunge [114] Kenyag | 84 children aged 10–28 mo | Maternal report. The duration of diarrhea was not presented. | Weekly surveillance | Microscopy (troph. or cysts) | 1227 | 537 | 78.8% | 68.6% | 1.69 (1.15–2.54) |
| Veenemans [30] Tanzaniah | 558, age 6–60 mo | Diarrhea: any report by the caretaker or ≥3 stools in 24 h. The duration of diarrhea was not presented. | Health-facility based surveillance | EIA | Positive children: 192 | Negative children: 336 | Overall: 0.43 Micro nutrients: 0.58 No micro nutrients: 0.29 | 0.68 0.63 0.72 | 0.84 (.64–1.09) 1.04 (.75–1.43) 0.56 (.34–.90) |
| Valentiner-Branth [120] Guinea-Bissaui | 200 newborns followed from birth to age 2 y | Maternal report. The duration of episode was not presented. | Weekly home visits | Microscopy | na | na | na | na | 0.64 (.46–.89) |
Abbreviations: CI, confidence interval; EIA, enzyme immunoassay; na, not available; OR, odds ratio; RR, rate ratio; Troph, trophozoites.
a ORs and CIs were calculated using the raw data presented in the original manuscripts for studies that did not provide measurement of association [24, 110–114, 116, 118, 119]. The measurement of association was provided in the study of Baqui et al [117] (OR) Molbak et al [115] (multivariable adjusted OR), Fraser et al [123] (OR), Bilenko et al [29] (age-adjusted Mantel-Haenszel OR), Hollm-Delgado et al [27] (multivariable adjusted RR), Boeke et al [121] (multivariable adjusted incidence RR), Veenemans et al [30] (adjusted hazard ratio), Valentiner-Branth et al [120] (multivariable adjusted OR).
b OR was calculated for acute diarrhea and for persistent diarrhea separately, whereas the comparison group was nondiarrhea.
c Adjusted RR for the incidence of diarrheal episodes in G. lamblia–positive weeks as compared with G. lamblia–negative weeks.
d In the study of Boeke et al [121], the incidence of diarrhea days was calculated by dividing the total number of diarrhea days by child years of observation in children who were positive and negative for Giardia.
e This study [29] presented 2 analyses; this analysis reflects the detection rates of G. lamblia in diarrhea stools compared with nondiarrheal stools. Please see the second analysis in the next row.
f This study [29] presented 2 analyses; this analysis reflects the adjusted RR for the incidence of diarrheal episodes in G. lamblia–positive months as compared with G. lamblia–negative months. The first analysis is presented in the previous row.
g In the study of Chunge et al [114], the results reflect the detection of G. lamblia in stools in relation to maternal reports on diarrhea.
h In the study of Veenemans et al [30], the incidence of diarrheal episodes was calculated as the number of episodes divided by child-years of follow-up in children who tested positive and negative for G. lamblia at baseline. The results reported in this table are for any reported diarrhea.
i Valentiner-Branth et al [120], reported the odds ratio of maternal report on diarrhea during weekly home visits in which stool samples were collected if the child had or did not have diarrhea. The OR in this study reflect the odds of diarrhea during infection with Giardia.
Cohort Studies That Addressed the Role of Giardia lamblia in Diarrhea
| Study & Country . | Study Population . | Definition of Diarrhea . | Surveillance of Diarrhea . | Detection of Giardia . | No. Diarrheal Stools . | No. Nondiarrheal Stools . | G. lamblia– Positive Diarrhea Stools, % . | G. lamblia– Positive Nondiarrhea Stools, % . | OR/RR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|
| Guerrant [110] Brazil | 297, household members, all ages | A significant change in bowel habits: decreased consistency or increased frequency. The duration of diarrhea was presented for other pathogens than Giardia. | Daily surveillance was conducted by the mother, and by weekly home visits performed by research assistants. | Microscopy | 150 | 32 | 6.7% | 12.5% | 0.50 (.13–2.35) |
| Schorling [113] Brazilb | 175, age <5 y | An increase in stool frequency or decrease in consistency, which lasted ≥1 d and was separated from another episode by 3 diarrhea-free days. Acute diarrhea <14 d. Persistent diarrhea ≥14 d, | Home visits 3 times/wk | Microscopy | Acute diarrhea: 50, persistent diarrhea, 40 | 38 | Acute diarrhea: 22%, persistent diarrhea:17.5% | 13.2% | 1.86 (.59–6.45) 1.40 (.39–5.26) |
| Newman [111] Brazil | 157 newborns followed up from birth | ≥3 unformed stools in 24 h. Acute diarrhea lasting <14 d. Persistent diarrhea ≥14 d. | Home visits 3 times/wk. | Microscopy | Acute diarrhea: 514, persistent diarrhea: 97 | 299 | Acute diarrhea: 7.6%, persistent diarrhea: 20.6% | 7.4% | 1.03 (.58–1.84) 3.27 (1.62–6.62) |
| Black [116] Peru | 153 newborns followed from birth | ≥1 d with liquid stools totaling 6 for infants <1 mo, 5 for infants aged 1 mo & 4 for older infants. New episode began after 2 free-illness days. The duration of diarrhea was presented for other pathogens than Giardia | Thrice-weekly home visits | Microscopy | 952 | 1973 | 0.7% | 0.8% | 0.91 (.35–2.18) |
| Kaminsky [119] Honduras | 266, 101 controls, Age <6 y | An increase in the usual number & change in the consistency of stools for ≥1 d. Acute & persistent diarrhea | Twice-weekly visits | Microscopy (troph. or cysts) | 848 | 101 | 29% | 57% | 0.50 (.42–.63) |
| Hollm-Delgado [27] Peruc | 220 infants followed up from birth to age 35 mo | ≥3 liquid/semi liquid stools/d in 2 consecutive days. The duration of diarrhea was not presented. | Daily home visits | Microscopy | 3911 | 16 973 | 6% | 6.2% | 0.95 (.79–1.13) |
| Boeke [121] Colombiad | 442, age 5–12 y | Maternal reports. The outcome was diarrhea days. | Daily reports in pictorial diaries | Microscopy (cysts) | Positive children: 28 | Negative children: 414 | 4.0 | 4.7 | 0.73 (.52–1.02) |
| Stanton [24] Bangladesh | 343, age <6 y | ≥ 3 loose stools in 24 h; new episode began after 14 d without diarrhea. Duration of diarrhea was not presented. | Fortnightly maternal interviews | Microscopy (cysts) | 225 | 1006 | 11% | 4% | 2.61 (1.53–4.37) |
| Baqui [117] Bangladesh | 705, age <5 y | ≥3 liquid/loose or watery stools or at least 1 bloody stool in 24-hours, Acute diarrhea <14 d, persistent diarrhea ≥4 d | Home visits every fourth day. | Microscopy | Acute diarrhea: 161 Persistent diarrhea: 167 | 165 | Acute diarrhea: 0.6% Persistent diarrhea: 1.2% | 1.8% | 0.33 0.68 |
| Hasan [118] Bangladesh | 252 newborns followed from birth for 2 y | ≥3 liquid stools in 24 h or any loose stools accompanied with blood in 24 h. Acute diarrhea <2 wk. Persistent diarrhea ≥2 wk. Data for Giardia were presented in a pooled analysis of acute & persistent diarrhea. | Twice-weekly home visits | Microscopy (troph.) | 1748 | 5679 | 13.2% | 13% | 1.01 (.86–1.19) |
| Zaki [112] Egypt | 2563, household members, all ages | Reports of the family speak person. The duration of diarrhea was not presented | Twice-weekly home visits | Microscopy | 3080 | 703 | 44.3% | 56.0% | 0.63 (.53–.74) |
| Fraser [123] Israel | 164 Bedouin newborns followed from birth to age 23 mo | ≥3 soft stools in 24 hours. For infants aged <1 mo ≥4 soft stools. The duration of diarrhea was not presented. | Through the local clinics and hospital, and through monthly and weekly maternal interviews. | Microscopy (cysts) | 239 | 730 | 22.3% | 28.5% | 0.8 (.7–.9) |
| Bilenko [29] Israele | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 349 | 8591 | 16% | 23% | 0.65 (.47–.91) |
| Bilenko [29] Israelf | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 1453 Giardia-positive months | 3001 Giardia-negative months | 6.7% | 6.7% | 1.09 (.81–1.46) |
| Molbak [115] Guinea-Bissau | 471–755 children | Maternal reports. Data for Giardia were presented in a pooled analysis of acute and persistent diarrhea. | Weekly visits | Microscopy (troph. or cysts) Troph. | 1219 | 511 | 19.1%, 9.3% | 25%, 9.8% | 0.8 (.6–1.0) 1.1 (.7–1.5) |
| Chunge [114] Kenyag | 84 children aged 10–28 mo | Maternal report. The duration of diarrhea was not presented. | Weekly surveillance | Microscopy (troph. or cysts) | 1227 | 537 | 78.8% | 68.6% | 1.69 (1.15–2.54) |
| Veenemans [30] Tanzaniah | 558, age 6–60 mo | Diarrhea: any report by the caretaker or ≥3 stools in 24 h. The duration of diarrhea was not presented. | Health-facility based surveillance | EIA | Positive children: 192 | Negative children: 336 | Overall: 0.43 Micro nutrients: 0.58 No micro nutrients: 0.29 | 0.68 0.63 0.72 | 0.84 (.64–1.09) 1.04 (.75–1.43) 0.56 (.34–.90) |
| Valentiner-Branth [120] Guinea-Bissaui | 200 newborns followed from birth to age 2 y | Maternal report. The duration of episode was not presented. | Weekly home visits | Microscopy | na | na | na | na | 0.64 (.46–.89) |
| Study & Country . | Study Population . | Definition of Diarrhea . | Surveillance of Diarrhea . | Detection of Giardia . | No. Diarrheal Stools . | No. Nondiarrheal Stools . | G. lamblia– Positive Diarrhea Stools, % . | G. lamblia– Positive Nondiarrhea Stools, % . | OR/RR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|
| Guerrant [110] Brazil | 297, household members, all ages | A significant change in bowel habits: decreased consistency or increased frequency. The duration of diarrhea was presented for other pathogens than Giardia. | Daily surveillance was conducted by the mother, and by weekly home visits performed by research assistants. | Microscopy | 150 | 32 | 6.7% | 12.5% | 0.50 (.13–2.35) |
| Schorling [113] Brazilb | 175, age <5 y | An increase in stool frequency or decrease in consistency, which lasted ≥1 d and was separated from another episode by 3 diarrhea-free days. Acute diarrhea <14 d. Persistent diarrhea ≥14 d, | Home visits 3 times/wk | Microscopy | Acute diarrhea: 50, persistent diarrhea, 40 | 38 | Acute diarrhea: 22%, persistent diarrhea:17.5% | 13.2% | 1.86 (.59–6.45) 1.40 (.39–5.26) |
| Newman [111] Brazil | 157 newborns followed up from birth | ≥3 unformed stools in 24 h. Acute diarrhea lasting <14 d. Persistent diarrhea ≥14 d. | Home visits 3 times/wk. | Microscopy | Acute diarrhea: 514, persistent diarrhea: 97 | 299 | Acute diarrhea: 7.6%, persistent diarrhea: 20.6% | 7.4% | 1.03 (.58–1.84) 3.27 (1.62–6.62) |
| Black [116] Peru | 153 newborns followed from birth | ≥1 d with liquid stools totaling 6 for infants <1 mo, 5 for infants aged 1 mo & 4 for older infants. New episode began after 2 free-illness days. The duration of diarrhea was presented for other pathogens than Giardia | Thrice-weekly home visits | Microscopy | 952 | 1973 | 0.7% | 0.8% | 0.91 (.35–2.18) |
| Kaminsky [119] Honduras | 266, 101 controls, Age <6 y | An increase in the usual number & change in the consistency of stools for ≥1 d. Acute & persistent diarrhea | Twice-weekly visits | Microscopy (troph. or cysts) | 848 | 101 | 29% | 57% | 0.50 (.42–.63) |
| Hollm-Delgado [27] Peruc | 220 infants followed up from birth to age 35 mo | ≥3 liquid/semi liquid stools/d in 2 consecutive days. The duration of diarrhea was not presented. | Daily home visits | Microscopy | 3911 | 16 973 | 6% | 6.2% | 0.95 (.79–1.13) |
| Boeke [121] Colombiad | 442, age 5–12 y | Maternal reports. The outcome was diarrhea days. | Daily reports in pictorial diaries | Microscopy (cysts) | Positive children: 28 | Negative children: 414 | 4.0 | 4.7 | 0.73 (.52–1.02) |
| Stanton [24] Bangladesh | 343, age <6 y | ≥ 3 loose stools in 24 h; new episode began after 14 d without diarrhea. Duration of diarrhea was not presented. | Fortnightly maternal interviews | Microscopy (cysts) | 225 | 1006 | 11% | 4% | 2.61 (1.53–4.37) |
| Baqui [117] Bangladesh | 705, age <5 y | ≥3 liquid/loose or watery stools or at least 1 bloody stool in 24-hours, Acute diarrhea <14 d, persistent diarrhea ≥4 d | Home visits every fourth day. | Microscopy | Acute diarrhea: 161 Persistent diarrhea: 167 | 165 | Acute diarrhea: 0.6% Persistent diarrhea: 1.2% | 1.8% | 0.33 0.68 |
| Hasan [118] Bangladesh | 252 newborns followed from birth for 2 y | ≥3 liquid stools in 24 h or any loose stools accompanied with blood in 24 h. Acute diarrhea <2 wk. Persistent diarrhea ≥2 wk. Data for Giardia were presented in a pooled analysis of acute & persistent diarrhea. | Twice-weekly home visits | Microscopy (troph.) | 1748 | 5679 | 13.2% | 13% | 1.01 (.86–1.19) |
| Zaki [112] Egypt | 2563, household members, all ages | Reports of the family speak person. The duration of diarrhea was not presented | Twice-weekly home visits | Microscopy | 3080 | 703 | 44.3% | 56.0% | 0.63 (.53–.74) |
| Fraser [123] Israel | 164 Bedouin newborns followed from birth to age 23 mo | ≥3 soft stools in 24 hours. For infants aged <1 mo ≥4 soft stools. The duration of diarrhea was not presented. | Through the local clinics and hospital, and through monthly and weekly maternal interviews. | Microscopy (cysts) | 239 | 730 | 22.3% | 28.5% | 0.8 (.7–.9) |
| Bilenko [29] Israele | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 349 | 8591 | 16% | 23% | 0.65 (.47–.91) |
| Bilenko [29] Israelf | 238 Bedouin newborns followed from birth to age 23 mo | Maternal reports. The duration of diarrhea (acute vs persistent was not presented). | Weekly maternal interviews | EIA | 1453 Giardia-positive months | 3001 Giardia-negative months | 6.7% | 6.7% | 1.09 (.81–1.46) |
| Molbak [115] Guinea-Bissau | 471–755 children | Maternal reports. Data for Giardia were presented in a pooled analysis of acute and persistent diarrhea. | Weekly visits | Microscopy (troph. or cysts) Troph. | 1219 | 511 | 19.1%, 9.3% | 25%, 9.8% | 0.8 (.6–1.0) 1.1 (.7–1.5) |
| Chunge [114] Kenyag | 84 children aged 10–28 mo | Maternal report. The duration of diarrhea was not presented. | Weekly surveillance | Microscopy (troph. or cysts) | 1227 | 537 | 78.8% | 68.6% | 1.69 (1.15–2.54) |
| Veenemans [30] Tanzaniah | 558, age 6–60 mo | Diarrhea: any report by the caretaker or ≥3 stools in 24 h. The duration of diarrhea was not presented. | Health-facility based surveillance | EIA | Positive children: 192 | Negative children: 336 | Overall: 0.43 Micro nutrients: 0.58 No micro nutrients: 0.29 | 0.68 0.63 0.72 | 0.84 (.64–1.09) 1.04 (.75–1.43) 0.56 (.34–.90) |
| Valentiner-Branth [120] Guinea-Bissaui | 200 newborns followed from birth to age 2 y | Maternal report. The duration of episode was not presented. | Weekly home visits | Microscopy | na | na | na | na | 0.64 (.46–.89) |
Abbreviations: CI, confidence interval; EIA, enzyme immunoassay; na, not available; OR, odds ratio; RR, rate ratio; Troph, trophozoites.
a ORs and CIs were calculated using the raw data presented in the original manuscripts for studies that did not provide measurement of association [24, 110–114, 116, 118, 119]. The measurement of association was provided in the study of Baqui et al [117] (OR) Molbak et al [115] (multivariable adjusted OR), Fraser et al [123] (OR), Bilenko et al [29] (age-adjusted Mantel-Haenszel OR), Hollm-Delgado et al [27] (multivariable adjusted RR), Boeke et al [121] (multivariable adjusted incidence RR), Veenemans et al [30] (adjusted hazard ratio), Valentiner-Branth et al [120] (multivariable adjusted OR).
b OR was calculated for acute diarrhea and for persistent diarrhea separately, whereas the comparison group was nondiarrhea.
c Adjusted RR for the incidence of diarrheal episodes in G. lamblia–positive weeks as compared with G. lamblia–negative weeks.
d In the study of Boeke et al [121], the incidence of diarrhea days was calculated by dividing the total number of diarrhea days by child years of observation in children who were positive and negative for Giardia.
e This study [29] presented 2 analyses; this analysis reflects the detection rates of G. lamblia in diarrhea stools compared with nondiarrheal stools. Please see the second analysis in the next row.
f This study [29] presented 2 analyses; this analysis reflects the adjusted RR for the incidence of diarrheal episodes in G. lamblia–positive months as compared with G. lamblia–negative months. The first analysis is presented in the previous row.
g In the study of Chunge et al [114], the results reflect the detection of G. lamblia in stools in relation to maternal reports on diarrhea.
h In the study of Veenemans et al [30], the incidence of diarrheal episodes was calculated as the number of episodes divided by child-years of follow-up in children who tested positive and negative for G. lamblia at baseline. The results reported in this table are for any reported diarrhea.
i Valentiner-Branth et al [120], reported the odds ratio of maternal report on diarrhea during weekly home visits in which stool samples were collected if the child had or did not have diarrhea. The OR in this study reflect the odds of diarrhea during infection with Giardia.
The analytical approach compared the prevalence of G. lamblia in stool samples that were obtained during diarrheal episodes with the prevalence of the parasite in stools obtained from asymptomatic children or in nondiarrheal stools that were obtained on a systematic predetermined basis (routine surveillance). Two studies compared the incidence of diarrhea in “G. lamblia–positive periods” with “G. lamblia–negative periods” [27, 29]. In 2 studies the incidence of diarrhea was compared between children who were positive for G. lamblia and children whose stool was negative for G. lamblia [30, 121]. Measurements of association were reported in only a fraction of the studies [27, 29, 30, 120, 121, 123]. Age- or multivariable-adjusted results were presented in 7 studies [27, 29, 30, 115, 117, 120, 121], whereas the rest presented unadjusted data [24, 111–113, 116, 122, 123]. In some studies, children with diarrhea who provided stool samples were matched with asymptomatic children who delivered stools during routine surveillance, for comparison [112, 113, 115, 117].
Many of the studies did not present the duration of diarrhea. Some studies presented a pooled analysis of acute and persistent diarrhea and a few presented separate analyses for acute vs persistent diarrhea (Table 2).
Only 3 of the 18 cohort studies showed a significantly increased risk of diarrhea in subjects infected with G. lamblia [24, 111, 114], while 7 studies showed no significant association between Giardia and diarrhea [27, 110, 113, 116–118, 121] (Table 2). One study investigated the length of carriage of Giardia in relation to the occurrence of diarrhea but found no significant association [122]. Interestingly, 7 cohort studies actually showed a lower risk for diarrhea in relation to the presence of G. lamblia in stools [29, 30, 112, 115, 119, 120, 123].
Is the Association Between G. lamblia and Diarrhea Age-Dependent?
We hypothesized that the association between G. lamblia and acute pediatric diarrhea among children in developing countries might be age-dependent; that is, the first infections that occur early in life might be associated with clinical diarrhea, whereas Giardia infections in older children might be largely asymptomatic (or may even lower the risk of acute diarrhea).
To address this hypothesis in this review, we abstracted data from studies that presented age-stratified results of the association between G. lamblia and diarrhea [24, 27–29, 70, 80, 118, 123]. An impediment to successful pursuit of this analysis was the heterogeneity of the age strata used for reporting data in the different studies (Table 3). Among these, one study reported a significant increased risk for diarrhea among Giardia-infected subjects in the youngest age group (<3 months of age) and a lower risk or no association between Giardia and diarrhea in older ages [123] (Table 3).
Association Between Giardia lamblia Infection and Diarrhea by Age Groups
| Study & Country . | Age Groups (mo) . | No. Giardia Positive/No. Diarrhea . | No. Giardia Positive/No. Controls . | OR (95% CI)a . |
|---|---|---|---|---|
| Loening [28] South Africa | 0–6 | 1.2% (n = 80) | 1.7% (n = 58) | 0.72 (.02–27.05) |
| 7–12 | 7.1% (n = 113) | 4.3% (n = 115) | 1.68 (.52–5.78) | |
| 13–24 | 8.5% (n = 130) | 6.5% (n = 124) | 1.34 (.51–3.60) | |
| 25–72 | 8% (n = 50) | 10.8% (n = 74) | 0.72 (.18–2.53) | |
| Fraser [123] Israel | ≤3 | 4.2% | 1.1% | 4.1 (1.1–15.3) |
| 4–6 | 5.2% | 3.2% | 1.6 (.6–4.2) | |
| 7–9 | 8.7% | 11.1% | 0.8 (.4–1.4) | |
| 10–12 | 13.4% | 23% | 0.5 (.3–0.8) | |
| 13–15 | 31.8% | 33.8% | 0.9 (.6–1.3) | |
| 16–18 | 27.8% | 35.9% | 0.7 (.4–1.1) | |
| 19–21 | 41.4% | 37% | 1.2 (.8–1.9) | |
| 22–24 | 37.9% | 36.1% | 1.0 (.6–1.9) | |
| Stanton [24] Bangladesh | <12 | 3/38 (8%) | 0/131 (0%) | … |
| 12–23 | 4/55 (7%) | 12/173 (7%) | 1.05 (.28–3.30) | |
| 24–72 | 17/132 (13%) | 32/702 (5%) | 3.10 (1.63–5.72) | |
| Hasan [118] Bangladesh | 0–5 | 2.7% (n = 300) | 2.6% (n = 1429) | 1.03 (.45–2.16) |
| 6–11 | 9.2% (n = 532) | 7.3% (n = 1382) | 1.29 (.89–1.83) | |
| 12–17 | 16.5% (n = 520) | 16.9% (n = 1405) | 0.98 (.74–1.28) | |
| 18–23 | 22.2% (n = 396) | 25.1% (n = 1463) | 0.85 (.65–1.11) | |
| Haque [80] Bangladesh | 0–12 mo | 38/1088 (3.5%) | 18/485 (3.7%) | 0.94 (.53–1.70) |
| 1–5 y | 4/672 (6.1%) | 160/660 (24.2%) | 0.20 (.14–0.29) | |
| 6–14 y | 31/279 (11.1%) | 146/457 (31.9%) | 0.27 (.17–0.40) | |
| 15–40 y | 91/1222 (7.4%) | 92/753 (12.2%) | 0.58 (.43–0.79) | |
| >40 y | 4/385 (1%) | 24/220 (10.9%) | 0.09 (.03–0.24) | |
| Bodhidatta [70] Thailand | 3–12 | 5/85 (6%) | 10/103 (10%) | 0.58 (.17–1.77) |
| 13–24 | 12/79 (15%) | 28/77 (36%) | 0.31 (.14–0.68) | |
| 25–59 | 14/43 (33%) | 15/47 (32%) | 1.00 (.42–2.53) | |
| Studies on the incidence of diarrhea in Giardia-positive and -negative periods | ||||
| No. diarrheal episodes/Giardia-positive periods | No. diarrheal episodes/Giardia-negative periods | OR/RR (95% CI) | ||
| Bilenko [29] Israelb | 0–6 | 3/99 | 100/1565 | 0.46 (.11–1.53) |
| 7–12 | 45/508 | 80/914 | 1.01 (.68–1.51) | |
| 13–18 | 50/846 | 20/522 | 1.58 (.90–2.78) | |
| Hollm-Delgado [27] Peruc | 0–5 | 10/188 | 157/4404 | 1.56 (.7–3.3) |
| 6–11 | 25/402 | 319/4321 | 0.86 (.6–1.2) | |
| 12–17 | 70/762 | 298/3291 | 1.02 (.8–1.4) | |
| 18–23 | 49/901 | 146/2289 | 1.00 (.7–1.4) | |
| 24–35 | 81/1658 | 135/2668 | 0.94 (.7–1.4) |
| Study & Country . | Age Groups (mo) . | No. Giardia Positive/No. Diarrhea . | No. Giardia Positive/No. Controls . | OR (95% CI)a . |
|---|---|---|---|---|
| Loening [28] South Africa | 0–6 | 1.2% (n = 80) | 1.7% (n = 58) | 0.72 (.02–27.05) |
| 7–12 | 7.1% (n = 113) | 4.3% (n = 115) | 1.68 (.52–5.78) | |
| 13–24 | 8.5% (n = 130) | 6.5% (n = 124) | 1.34 (.51–3.60) | |
| 25–72 | 8% (n = 50) | 10.8% (n = 74) | 0.72 (.18–2.53) | |
| Fraser [123] Israel | ≤3 | 4.2% | 1.1% | 4.1 (1.1–15.3) |
| 4–6 | 5.2% | 3.2% | 1.6 (.6–4.2) | |
| 7–9 | 8.7% | 11.1% | 0.8 (.4–1.4) | |
| 10–12 | 13.4% | 23% | 0.5 (.3–0.8) | |
| 13–15 | 31.8% | 33.8% | 0.9 (.6–1.3) | |
| 16–18 | 27.8% | 35.9% | 0.7 (.4–1.1) | |
| 19–21 | 41.4% | 37% | 1.2 (.8–1.9) | |
| 22–24 | 37.9% | 36.1% | 1.0 (.6–1.9) | |
| Stanton [24] Bangladesh | <12 | 3/38 (8%) | 0/131 (0%) | … |
| 12–23 | 4/55 (7%) | 12/173 (7%) | 1.05 (.28–3.30) | |
| 24–72 | 17/132 (13%) | 32/702 (5%) | 3.10 (1.63–5.72) | |
| Hasan [118] Bangladesh | 0–5 | 2.7% (n = 300) | 2.6% (n = 1429) | 1.03 (.45–2.16) |
| 6–11 | 9.2% (n = 532) | 7.3% (n = 1382) | 1.29 (.89–1.83) | |
| 12–17 | 16.5% (n = 520) | 16.9% (n = 1405) | 0.98 (.74–1.28) | |
| 18–23 | 22.2% (n = 396) | 25.1% (n = 1463) | 0.85 (.65–1.11) | |
| Haque [80] Bangladesh | 0–12 mo | 38/1088 (3.5%) | 18/485 (3.7%) | 0.94 (.53–1.70) |
| 1–5 y | 4/672 (6.1%) | 160/660 (24.2%) | 0.20 (.14–0.29) | |
| 6–14 y | 31/279 (11.1%) | 146/457 (31.9%) | 0.27 (.17–0.40) | |
| 15–40 y | 91/1222 (7.4%) | 92/753 (12.2%) | 0.58 (.43–0.79) | |
| >40 y | 4/385 (1%) | 24/220 (10.9%) | 0.09 (.03–0.24) | |
| Bodhidatta [70] Thailand | 3–12 | 5/85 (6%) | 10/103 (10%) | 0.58 (.17–1.77) |
| 13–24 | 12/79 (15%) | 28/77 (36%) | 0.31 (.14–0.68) | |
| 25–59 | 14/43 (33%) | 15/47 (32%) | 1.00 (.42–2.53) | |
| Studies on the incidence of diarrhea in Giardia-positive and -negative periods | ||||
| No. diarrheal episodes/Giardia-positive periods | No. diarrheal episodes/Giardia-negative periods | OR/RR (95% CI) | ||
| Bilenko [29] Israelb | 0–6 | 3/99 | 100/1565 | 0.46 (.11–1.53) |
| 7–12 | 45/508 | 80/914 | 1.01 (.68–1.51) | |
| 13–18 | 50/846 | 20/522 | 1.58 (.90–2.78) | |
| Hollm-Delgado [27] Peruc | 0–5 | 10/188 | 157/4404 | 1.56 (.7–3.3) |
| 6–11 | 25/402 | 319/4321 | 0.86 (.6–1.2) | |
| 12–17 | 70/762 | 298/3291 | 1.02 (.8–1.4) | |
| 18–23 | 49/901 | 146/2289 | 1.00 (.7–1.4) | |
| 24–35 | 81/1658 | 135/2668 | 0.94 (.7–1.4) |
Abbreviations: CI, confidence interval; OR, odds ratio; RR, rate ratio.
a We calculated the ORs and 95% CIs for Bodhidatta et al [70], Hasan et al [118], Stanton et al [24], and Haque et al [80].
b Bilenko et al [29] presented data on the number of diarrheal episodes in months in which G. lamblia was detected compared with months in which G. lamblia was not detected, and presented OR.
c Hollm-Delgado et al [27] presented data on diarrheal stools that were positive for Giardia among all Giardia-positive stools, compared with diarrheal stools that were negative for Giardia among all Giardia-negative stools, and presented the adjusted RR.
Association Between Giardia lamblia Infection and Diarrhea by Age Groups
| Study & Country . | Age Groups (mo) . | No. Giardia Positive/No. Diarrhea . | No. Giardia Positive/No. Controls . | OR (95% CI)a . |
|---|---|---|---|---|
| Loening [28] South Africa | 0–6 | 1.2% (n = 80) | 1.7% (n = 58) | 0.72 (.02–27.05) |
| 7–12 | 7.1% (n = 113) | 4.3% (n = 115) | 1.68 (.52–5.78) | |
| 13–24 | 8.5% (n = 130) | 6.5% (n = 124) | 1.34 (.51–3.60) | |
| 25–72 | 8% (n = 50) | 10.8% (n = 74) | 0.72 (.18–2.53) | |
| Fraser [123] Israel | ≤3 | 4.2% | 1.1% | 4.1 (1.1–15.3) |
| 4–6 | 5.2% | 3.2% | 1.6 (.6–4.2) | |
| 7–9 | 8.7% | 11.1% | 0.8 (.4–1.4) | |
| 10–12 | 13.4% | 23% | 0.5 (.3–0.8) | |
| 13–15 | 31.8% | 33.8% | 0.9 (.6–1.3) | |
| 16–18 | 27.8% | 35.9% | 0.7 (.4–1.1) | |
| 19–21 | 41.4% | 37% | 1.2 (.8–1.9) | |
| 22–24 | 37.9% | 36.1% | 1.0 (.6–1.9) | |
| Stanton [24] Bangladesh | <12 | 3/38 (8%) | 0/131 (0%) | … |
| 12–23 | 4/55 (7%) | 12/173 (7%) | 1.05 (.28–3.30) | |
| 24–72 | 17/132 (13%) | 32/702 (5%) | 3.10 (1.63–5.72) | |
| Hasan [118] Bangladesh | 0–5 | 2.7% (n = 300) | 2.6% (n = 1429) | 1.03 (.45–2.16) |
| 6–11 | 9.2% (n = 532) | 7.3% (n = 1382) | 1.29 (.89–1.83) | |
| 12–17 | 16.5% (n = 520) | 16.9% (n = 1405) | 0.98 (.74–1.28) | |
| 18–23 | 22.2% (n = 396) | 25.1% (n = 1463) | 0.85 (.65–1.11) | |
| Haque [80] Bangladesh | 0–12 mo | 38/1088 (3.5%) | 18/485 (3.7%) | 0.94 (.53–1.70) |
| 1–5 y | 4/672 (6.1%) | 160/660 (24.2%) | 0.20 (.14–0.29) | |
| 6–14 y | 31/279 (11.1%) | 146/457 (31.9%) | 0.27 (.17–0.40) | |
| 15–40 y | 91/1222 (7.4%) | 92/753 (12.2%) | 0.58 (.43–0.79) | |
| >40 y | 4/385 (1%) | 24/220 (10.9%) | 0.09 (.03–0.24) | |
| Bodhidatta [70] Thailand | 3–12 | 5/85 (6%) | 10/103 (10%) | 0.58 (.17–1.77) |
| 13–24 | 12/79 (15%) | 28/77 (36%) | 0.31 (.14–0.68) | |
| 25–59 | 14/43 (33%) | 15/47 (32%) | 1.00 (.42–2.53) | |
| Studies on the incidence of diarrhea in Giardia-positive and -negative periods | ||||
| No. diarrheal episodes/Giardia-positive periods | No. diarrheal episodes/Giardia-negative periods | OR/RR (95% CI) | ||
| Bilenko [29] Israelb | 0–6 | 3/99 | 100/1565 | 0.46 (.11–1.53) |
| 7–12 | 45/508 | 80/914 | 1.01 (.68–1.51) | |
| 13–18 | 50/846 | 20/522 | 1.58 (.90–2.78) | |
| Hollm-Delgado [27] Peruc | 0–5 | 10/188 | 157/4404 | 1.56 (.7–3.3) |
| 6–11 | 25/402 | 319/4321 | 0.86 (.6–1.2) | |
| 12–17 | 70/762 | 298/3291 | 1.02 (.8–1.4) | |
| 18–23 | 49/901 | 146/2289 | 1.00 (.7–1.4) | |
| 24–35 | 81/1658 | 135/2668 | 0.94 (.7–1.4) |
| Study & Country . | Age Groups (mo) . | No. Giardia Positive/No. Diarrhea . | No. Giardia Positive/No. Controls . | OR (95% CI)a . |
|---|---|---|---|---|
| Loening [28] South Africa | 0–6 | 1.2% (n = 80) | 1.7% (n = 58) | 0.72 (.02–27.05) |
| 7–12 | 7.1% (n = 113) | 4.3% (n = 115) | 1.68 (.52–5.78) | |
| 13–24 | 8.5% (n = 130) | 6.5% (n = 124) | 1.34 (.51–3.60) | |
| 25–72 | 8% (n = 50) | 10.8% (n = 74) | 0.72 (.18–2.53) | |
| Fraser [123] Israel | ≤3 | 4.2% | 1.1% | 4.1 (1.1–15.3) |
| 4–6 | 5.2% | 3.2% | 1.6 (.6–4.2) | |
| 7–9 | 8.7% | 11.1% | 0.8 (.4–1.4) | |
| 10–12 | 13.4% | 23% | 0.5 (.3–0.8) | |
| 13–15 | 31.8% | 33.8% | 0.9 (.6–1.3) | |
| 16–18 | 27.8% | 35.9% | 0.7 (.4–1.1) | |
| 19–21 | 41.4% | 37% | 1.2 (.8–1.9) | |
| 22–24 | 37.9% | 36.1% | 1.0 (.6–1.9) | |
| Stanton [24] Bangladesh | <12 | 3/38 (8%) | 0/131 (0%) | … |
| 12–23 | 4/55 (7%) | 12/173 (7%) | 1.05 (.28–3.30) | |
| 24–72 | 17/132 (13%) | 32/702 (5%) | 3.10 (1.63–5.72) | |
| Hasan [118] Bangladesh | 0–5 | 2.7% (n = 300) | 2.6% (n = 1429) | 1.03 (.45–2.16) |
| 6–11 | 9.2% (n = 532) | 7.3% (n = 1382) | 1.29 (.89–1.83) | |
| 12–17 | 16.5% (n = 520) | 16.9% (n = 1405) | 0.98 (.74–1.28) | |
| 18–23 | 22.2% (n = 396) | 25.1% (n = 1463) | 0.85 (.65–1.11) | |
| Haque [80] Bangladesh | 0–12 mo | 38/1088 (3.5%) | 18/485 (3.7%) | 0.94 (.53–1.70) |
| 1–5 y | 4/672 (6.1%) | 160/660 (24.2%) | 0.20 (.14–0.29) | |
| 6–14 y | 31/279 (11.1%) | 146/457 (31.9%) | 0.27 (.17–0.40) | |
| 15–40 y | 91/1222 (7.4%) | 92/753 (12.2%) | 0.58 (.43–0.79) | |
| >40 y | 4/385 (1%) | 24/220 (10.9%) | 0.09 (.03–0.24) | |
| Bodhidatta [70] Thailand | 3–12 | 5/85 (6%) | 10/103 (10%) | 0.58 (.17–1.77) |
| 13–24 | 12/79 (15%) | 28/77 (36%) | 0.31 (.14–0.68) | |
| 25–59 | 14/43 (33%) | 15/47 (32%) | 1.00 (.42–2.53) | |
| Studies on the incidence of diarrhea in Giardia-positive and -negative periods | ||||
| No. diarrheal episodes/Giardia-positive periods | No. diarrheal episodes/Giardia-negative periods | OR/RR (95% CI) | ||
| Bilenko [29] Israelb | 0–6 | 3/99 | 100/1565 | 0.46 (.11–1.53) |
| 7–12 | 45/508 | 80/914 | 1.01 (.68–1.51) | |
| 13–18 | 50/846 | 20/522 | 1.58 (.90–2.78) | |
| Hollm-Delgado [27] Peruc | 0–5 | 10/188 | 157/4404 | 1.56 (.7–3.3) |
| 6–11 | 25/402 | 319/4321 | 0.86 (.6–1.2) | |
| 12–17 | 70/762 | 298/3291 | 1.02 (.8–1.4) | |
| 18–23 | 49/901 | 146/2289 | 1.00 (.7–1.4) | |
| 24–35 | 81/1658 | 135/2668 | 0.94 (.7–1.4) |
Abbreviations: CI, confidence interval; OR, odds ratio; RR, rate ratio.
a We calculated the ORs and 95% CIs for Bodhidatta et al [70], Hasan et al [118], Stanton et al [24], and Haque et al [80].
b Bilenko et al [29] presented data on the number of diarrheal episodes in months in which G. lamblia was detected compared with months in which G. lamblia was not detected, and presented OR.
c Hollm-Delgado et al [27] presented data on diarrheal stools that were positive for Giardia among all Giardia-positive stools, compared with diarrheal stools that were negative for Giardia among all Giardia-negative stools, and presented the adjusted RR.
META-ANALYSIS OF G. LAMBLIA AND ENDEMIC PEDIATRIC DIARRHEA
The Association Between G. lamblia and Acute Diarrhea
Ten case/control studies [28, 68, 70, 72, 73, 80, 82, 86, 90, 98] and 2 cohort studies [113, 117] that enrolled children from developing countries or other nonindustrialized settings were included in the meta-analysis because their design and execution revealed no fundamental flaws (as explained in the Methods section). From the study of Haque et al [80], we abstracted data only on children aged ≤5 years. These 12 studies [28, 68, 70, 72, 73, 80, 82, 86, 90, 98, 113, 117] fulfilled the inclusion criteria of presenting the outcome variable of acute diarrhea, they matched or controlled for potential confounders, and the study lasted at least 1 year. Using the random effects models, the pooled OR was 0.60 (95% CI, .38–.94; P = .03) (Figure 1). This suggests that the presence of Giardia infection actually diminished the likelihood of having acute diarrhea among children from developing countries. The heterogeneity test was statistically significant; χ2 77.9 (P < .001), I2 85.9%.
Forest plot of studies on the association between Giardia lamblia infection and acute diarrhea among children from developing countries. The odds ratio (OR) and 95% confidence interval (CI) of each study included in the meta-analysis and the pooled OR and 95% CI obtained using the random effects model are presented. Squares and bars represent individual study OR and 95% CI. Diamond represents pooled OR and 95% CI.
The Association Between G. lamblia and Persistent Diarrhea
Two cohort studies [113, 117] and 3 case/control studies [106, 107, 109] that fulfilled the inclusion criterion of matching between cases and controls and that lasted ≥1 year presented data on persistent diarrhea as an outcome. Results from these 5 studies were combined using the random effects model. The pooled OR was 3.18 (95% CI, 1.50–6.76; P < .001), suggesting that G. lamblia infection significantly increases the likelihood of persistent diarrhea (Figure 2). The heterogeneity χ2 test was 7.22 (P = .125), I2 44.6%.
Forest plot of studies on the association between Giardia lamblia infection and persistent diarrhea among children from developing countries. The odds ratio (OR) and 95% confidence interval (CI) of each study included in the meta-analysis and the pooled OR and 95% CI obtained using the random effects model are presented. Squares and bars represent individual study OR and 95% CI. Diamond represents pooled OR and 95% CI.
Assessing the Potential of Publication Bias
Figure 3, which presents funnel plots of studies included in the meta-analysis on acute diarrhea, appears visually symmetrical. The Egger regression intercept of the meta-analysis on acute diarrhea studies was 0.32 (95% CI, −3.18 to 3.83; 2-tailed P = .83). These results provide no hint of publication bias. Figure 4 shows the cumulative meta-analysis of studies on acute diarrhea. There is no evidence that the addition of the small studies affected the direction of the association between G. lamblia infection and the likelihood of acute diarrhea in children from developing countries. The Egger regression intercept of the meta-analysis of studies on persistent diarrhea was –2.57 (95% CI, −8.93 to 3.79; 2-tailed P = .28).
Funnel plot of studies included in the meta-analysis on the association between Giardia lamblia infection and acute diarrhea. The log odds ratio (OR) of each study on the x-axis is plotted against the corresponding standard error on the y-axis. The studies are represented in the funnel plot as opened circles. The rhombus shape at the x-axis reflects the log of the pooled OR obtained by using the random effects model.
Cumulative meta-analysis of the association between Giardia lamblia and acute diarrhea among children from developing countries by study sample size. The change in the pooled odds ratio (OR) is described by adding studies according to their sample size, starting with the largest study. Squares and bars represent individual study OR and 95% CI. Diamond represents pooled OR and 95% CI.
G. LAMBLIA AND TRAVELERS' DIARRHEA
Early reports of the etiology of travelers' diarrhea [124–126] emphasized enterotoxigenic Escherichia coli as the most frequent pathogen, present in approximately 30% of cases [124]. A recent review showed G. lamblia in 1.3% and 1.6% of travelers with diarrhea whose destination was Latin America or Africa, respectively, in comparison with 6.2% and 5.7% of travelers to South and Southeast Asia [124]. Only a few studies investigated in parallel the presence of Giardia in stools of travelers who did not develop diarrhea (as controls). Our review considered only studies that included a control group of travelers without diarrhea. We identified 12 case/control [127–138] and 2 cohort studies [139, 140] that examined the role of Giardia in travelers' diarrhea. Characteristics of these studies are shown in Table 4. Giardia lamblia was detected by means of stool microscopy [128, 132–140], except for one study that used enzyme immunoassay [131] and 2 studies that did not specify their method for detecting G. lamblia [129, 130].
Studies That Addressed the Role of Giardia in Travelers' Diarrhea
| Study . | Country of Origin . | Definition of Outcome . | Sampling . | Age . | Travel Destination . | Giardia-Positive Cases/Total Cases (%) . | Giardia-Positive Controls/Total Controls (%) . | RR/OR (95% CI)a . | Matching/Adjusting . |
|---|---|---|---|---|---|---|---|---|---|
| Andersson [139] | Sweden | Gastrointestinal symptoms | Students who traveled to Leningrad | Adult students | Leningrad | 27/27 (100%) | 3/11 (27.3%) | 3.66 (1.75–10.26) | None |
| Brodsky [133] | US | Gastrointestinal symptoms | Tourists, CDC surveillance | All ages | Former Soviet Union | 83/153 (54.3%) | 8/153 (5.2%) | 21.49 (10.11–44.49) | None |
| Merson [140] | US, Canada, Netherlands, England | The occurrence between 12 h after arrival to Mexico City and 5 d after departure of any unformed stool not attributed to a preexisting condition plus ≥1 enteric symptom. Or ≥3 watery stools in 24 h | Physicians & their family members | Mainly adults | Mexico | 1/51 (2%) | 1/43 (2.3%) | 0.84 (.01–67.49) | None |
| DuPont [132] | US, Venezuela, Mexico | Acute diarrhea: unformed bowel movements at a daily rate twice of the usual rate plus ≥1 enteric symptom | University clinic | Adult students | Mexico | US 6% (total cases 77), LA 18% (total cases 18) | US 3% (total controls 67), LA 11% (total controls 27) | 2.26 (.43–17.20), 1.60 (.19–13.43) | Country of origin, length of stay in Mexico |
| Bolivar [134] | US, Venezuela, Mexico | Unformed bowel movement at daily rate twice that of the subject's usual rate & ≥1 other enteric symptom | University clinic | Adult students | Mexico | 3/91 (3.3%) | 2/74 (2.7%) | 1.23 (.18–10.54) | Country of origin, length of stay in Mexico |
| Back [127] | Sweden | ≥2 abnormal loose stools/d | Swedish battalion in United Nations forces | Adults | Cyprus | 1/79 (1.3%) | 0/66 (0%) | Serving conditions (next bedfellow) | |
| Echeverria [135] | US | ≥3 loose stools or ≥2 loose stools with other enteric symptom | Soldiers who attended a clinic | Adults | Philippines | 3/152 (2%) | 2/58 (3.5%) | 0.56 (.06–6.94) | None |
| Hogeb [136] | Foreign residents & tourists | Change in normal bowel movements with ≥3 loose stools in 24 h | CIWEC, USEM | All ages | Nepal | 7/148 (4.7%) | 1/95 (1%) | 4.67 (.58–212.52) | Group matching by clinic & season |
| Shlim [128] | Tourists, expatriates | Change in normal bowel movements & ≥3 loose stools in 24 h | CIWEC | ≥18 y | Nepal | 25/189 (13.2%) | 3/112 (2.6%) | 5.54 (1.62–29.23) | None |
| Gascon [137] | Spain | Diarrhea that occurred between 12 h after arriving in & 5 d after departing from the travel country. Diarrhea ≥3 watery stools in 24 h, or unformed stools plus enteric symptom | Tropical Medicine Department | NA | Asia, Africa, Central & Latin America | 11/165 (6.7%) | 3/165 (1.8%) | 3.86 (.99–21.86) | Area visited, controls were relatives or travel companions of cases |
| Schultsz [138] | Netherlands | ≥3 loose stools in 24 h, any number of watery stools in 24 h, or 1–2 loose stools in 24 h plus ≥1 enteric symptom | Outpatient Department for Tropical Diseases | 2–75 y | Asia, Africa, Central & Latin America | Acute 2% (total cases 49), persistent 16.4% (total cases 116) | 4.9% (total controls 102) | 0.40 (.01–3.78), 3.80 (1.30–13.48) | None |
| Boggild [129] | Canada | Diagnosis of giardiasis | Tropical Disease Unit. (GeoSentinel Network) | Mean 37.3 y | International travel | 69/1622 (4.3%) | 5/1906 (0.3%) | 16.9 (6.8–41.9) | None |
| Paschke [130] | Germany | ≥3 unformed stools in 24 h plus ≥1 symptom of enteric infection | Department of Infectious Diseases & Tropical Medicine. | 2–80 y | Asia, Latin America, Europe, other | 7/114 (6.1%) | 3/56 (5.4%) | 1.16 (.25–7.20) | None |
| Pandey [131] | US, Japan, Australia, New Zealand, Western Europe | ≥3 unformed stools in 24 h | CIWEC | >18 y | Nepal | Overall 42/372 (11.3%) ≤7 d 7%, >7 d 26% | 5 (2.9%) | 3.75 (1.40–9.98), 2.48 (.95–7.52), 11.78 (4.41–35.90) | Age, sex, nationality, resident/tourist status, length of stay in Nepal, season |
| Study . | Country of Origin . | Definition of Outcome . | Sampling . | Age . | Travel Destination . | Giardia-Positive Cases/Total Cases (%) . | Giardia-Positive Controls/Total Controls (%) . | RR/OR (95% CI)a . | Matching/Adjusting . |
|---|---|---|---|---|---|---|---|---|---|
| Andersson [139] | Sweden | Gastrointestinal symptoms | Students who traveled to Leningrad | Adult students | Leningrad | 27/27 (100%) | 3/11 (27.3%) | 3.66 (1.75–10.26) | None |
| Brodsky [133] | US | Gastrointestinal symptoms | Tourists, CDC surveillance | All ages | Former Soviet Union | 83/153 (54.3%) | 8/153 (5.2%) | 21.49 (10.11–44.49) | None |
| Merson [140] | US, Canada, Netherlands, England | The occurrence between 12 h after arrival to Mexico City and 5 d after departure of any unformed stool not attributed to a preexisting condition plus ≥1 enteric symptom. Or ≥3 watery stools in 24 h | Physicians & their family members | Mainly adults | Mexico | 1/51 (2%) | 1/43 (2.3%) | 0.84 (.01–67.49) | None |
| DuPont [132] | US, Venezuela, Mexico | Acute diarrhea: unformed bowel movements at a daily rate twice of the usual rate plus ≥1 enteric symptom | University clinic | Adult students | Mexico | US 6% (total cases 77), LA 18% (total cases 18) | US 3% (total controls 67), LA 11% (total controls 27) | 2.26 (.43–17.20), 1.60 (.19–13.43) | Country of origin, length of stay in Mexico |
| Bolivar [134] | US, Venezuela, Mexico | Unformed bowel movement at daily rate twice that of the subject's usual rate & ≥1 other enteric symptom | University clinic | Adult students | Mexico | 3/91 (3.3%) | 2/74 (2.7%) | 1.23 (.18–10.54) | Country of origin, length of stay in Mexico |
| Back [127] | Sweden | ≥2 abnormal loose stools/d | Swedish battalion in United Nations forces | Adults | Cyprus | 1/79 (1.3%) | 0/66 (0%) | Serving conditions (next bedfellow) | |
| Echeverria [135] | US | ≥3 loose stools or ≥2 loose stools with other enteric symptom | Soldiers who attended a clinic | Adults | Philippines | 3/152 (2%) | 2/58 (3.5%) | 0.56 (.06–6.94) | None |
| Hogeb [136] | Foreign residents & tourists | Change in normal bowel movements with ≥3 loose stools in 24 h | CIWEC, USEM | All ages | Nepal | 7/148 (4.7%) | 1/95 (1%) | 4.67 (.58–212.52) | Group matching by clinic & season |
| Shlim [128] | Tourists, expatriates | Change in normal bowel movements & ≥3 loose stools in 24 h | CIWEC | ≥18 y | Nepal | 25/189 (13.2%) | 3/112 (2.6%) | 5.54 (1.62–29.23) | None |
| Gascon [137] | Spain | Diarrhea that occurred between 12 h after arriving in & 5 d after departing from the travel country. Diarrhea ≥3 watery stools in 24 h, or unformed stools plus enteric symptom | Tropical Medicine Department | NA | Asia, Africa, Central & Latin America | 11/165 (6.7%) | 3/165 (1.8%) | 3.86 (.99–21.86) | Area visited, controls were relatives or travel companions of cases |
| Schultsz [138] | Netherlands | ≥3 loose stools in 24 h, any number of watery stools in 24 h, or 1–2 loose stools in 24 h plus ≥1 enteric symptom | Outpatient Department for Tropical Diseases | 2–75 y | Asia, Africa, Central & Latin America | Acute 2% (total cases 49), persistent 16.4% (total cases 116) | 4.9% (total controls 102) | 0.40 (.01–3.78), 3.80 (1.30–13.48) | None |
| Boggild [129] | Canada | Diagnosis of giardiasis | Tropical Disease Unit. (GeoSentinel Network) | Mean 37.3 y | International travel | 69/1622 (4.3%) | 5/1906 (0.3%) | 16.9 (6.8–41.9) | None |
| Paschke [130] | Germany | ≥3 unformed stools in 24 h plus ≥1 symptom of enteric infection | Department of Infectious Diseases & Tropical Medicine. | 2–80 y | Asia, Latin America, Europe, other | 7/114 (6.1%) | 3/56 (5.4%) | 1.16 (.25–7.20) | None |
| Pandey [131] | US, Japan, Australia, New Zealand, Western Europe | ≥3 unformed stools in 24 h | CIWEC | >18 y | Nepal | Overall 42/372 (11.3%) ≤7 d 7%, >7 d 26% | 5 (2.9%) | 3.75 (1.40–9.98), 2.48 (.95–7.52), 11.78 (4.41–35.90) | Age, sex, nationality, resident/tourist status, length of stay in Nepal, season |
Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; LA, Latin American (Venezuela and Mexico); CIWEC, Canadian International Water and Energy Consultants; OR, odds ratio; RR, rate ratio; USEM, US Embassy Medical Care.
a ORs and 95% CIs were calculated using the abstracted data from each study. For the study of Andersson et al [139], RR was calculated. Boggild et al [129] reported crude OR and Pandey et al [131] reported adjusted OR.
b Data from the study of Hoge et al [136] were abstracted on 148 cases of diarrhea among which coccidian-like organisms were not identified. Cases and controls from both clinics (CIWEC and USEM) were pooled.
Studies That Addressed the Role of Giardia in Travelers' Diarrhea
| Study . | Country of Origin . | Definition of Outcome . | Sampling . | Age . | Travel Destination . | Giardia-Positive Cases/Total Cases (%) . | Giardia-Positive Controls/Total Controls (%) . | RR/OR (95% CI)a . | Matching/Adjusting . |
|---|---|---|---|---|---|---|---|---|---|
| Andersson [139] | Sweden | Gastrointestinal symptoms | Students who traveled to Leningrad | Adult students | Leningrad | 27/27 (100%) | 3/11 (27.3%) | 3.66 (1.75–10.26) | None |
| Brodsky [133] | US | Gastrointestinal symptoms | Tourists, CDC surveillance | All ages | Former Soviet Union | 83/153 (54.3%) | 8/153 (5.2%) | 21.49 (10.11–44.49) | None |
| Merson [140] | US, Canada, Netherlands, England | The occurrence between 12 h after arrival to Mexico City and 5 d after departure of any unformed stool not attributed to a preexisting condition plus ≥1 enteric symptom. Or ≥3 watery stools in 24 h | Physicians & their family members | Mainly adults | Mexico | 1/51 (2%) | 1/43 (2.3%) | 0.84 (.01–67.49) | None |
| DuPont [132] | US, Venezuela, Mexico | Acute diarrhea: unformed bowel movements at a daily rate twice of the usual rate plus ≥1 enteric symptom | University clinic | Adult students | Mexico | US 6% (total cases 77), LA 18% (total cases 18) | US 3% (total controls 67), LA 11% (total controls 27) | 2.26 (.43–17.20), 1.60 (.19–13.43) | Country of origin, length of stay in Mexico |
| Bolivar [134] | US, Venezuela, Mexico | Unformed bowel movement at daily rate twice that of the subject's usual rate & ≥1 other enteric symptom | University clinic | Adult students | Mexico | 3/91 (3.3%) | 2/74 (2.7%) | 1.23 (.18–10.54) | Country of origin, length of stay in Mexico |
| Back [127] | Sweden | ≥2 abnormal loose stools/d | Swedish battalion in United Nations forces | Adults | Cyprus | 1/79 (1.3%) | 0/66 (0%) | Serving conditions (next bedfellow) | |
| Echeverria [135] | US | ≥3 loose stools or ≥2 loose stools with other enteric symptom | Soldiers who attended a clinic | Adults | Philippines | 3/152 (2%) | 2/58 (3.5%) | 0.56 (.06–6.94) | None |
| Hogeb [136] | Foreign residents & tourists | Change in normal bowel movements with ≥3 loose stools in 24 h | CIWEC, USEM | All ages | Nepal | 7/148 (4.7%) | 1/95 (1%) | 4.67 (.58–212.52) | Group matching by clinic & season |
| Shlim [128] | Tourists, expatriates | Change in normal bowel movements & ≥3 loose stools in 24 h | CIWEC | ≥18 y | Nepal | 25/189 (13.2%) | 3/112 (2.6%) | 5.54 (1.62–29.23) | None |
| Gascon [137] | Spain | Diarrhea that occurred between 12 h after arriving in & 5 d after departing from the travel country. Diarrhea ≥3 watery stools in 24 h, or unformed stools plus enteric symptom | Tropical Medicine Department | NA | Asia, Africa, Central & Latin America | 11/165 (6.7%) | 3/165 (1.8%) | 3.86 (.99–21.86) | Area visited, controls were relatives or travel companions of cases |
| Schultsz [138] | Netherlands | ≥3 loose stools in 24 h, any number of watery stools in 24 h, or 1–2 loose stools in 24 h plus ≥1 enteric symptom | Outpatient Department for Tropical Diseases | 2–75 y | Asia, Africa, Central & Latin America | Acute 2% (total cases 49), persistent 16.4% (total cases 116) | 4.9% (total controls 102) | 0.40 (.01–3.78), 3.80 (1.30–13.48) | None |
| Boggild [129] | Canada | Diagnosis of giardiasis | Tropical Disease Unit. (GeoSentinel Network) | Mean 37.3 y | International travel | 69/1622 (4.3%) | 5/1906 (0.3%) | 16.9 (6.8–41.9) | None |
| Paschke [130] | Germany | ≥3 unformed stools in 24 h plus ≥1 symptom of enteric infection | Department of Infectious Diseases & Tropical Medicine. | 2–80 y | Asia, Latin America, Europe, other | 7/114 (6.1%) | 3/56 (5.4%) | 1.16 (.25–7.20) | None |
| Pandey [131] | US, Japan, Australia, New Zealand, Western Europe | ≥3 unformed stools in 24 h | CIWEC | >18 y | Nepal | Overall 42/372 (11.3%) ≤7 d 7%, >7 d 26% | 5 (2.9%) | 3.75 (1.40–9.98), 2.48 (.95–7.52), 11.78 (4.41–35.90) | Age, sex, nationality, resident/tourist status, length of stay in Nepal, season |
| Study . | Country of Origin . | Definition of Outcome . | Sampling . | Age . | Travel Destination . | Giardia-Positive Cases/Total Cases (%) . | Giardia-Positive Controls/Total Controls (%) . | RR/OR (95% CI)a . | Matching/Adjusting . |
|---|---|---|---|---|---|---|---|---|---|
| Andersson [139] | Sweden | Gastrointestinal symptoms | Students who traveled to Leningrad | Adult students | Leningrad | 27/27 (100%) | 3/11 (27.3%) | 3.66 (1.75–10.26) | None |
| Brodsky [133] | US | Gastrointestinal symptoms | Tourists, CDC surveillance | All ages | Former Soviet Union | 83/153 (54.3%) | 8/153 (5.2%) | 21.49 (10.11–44.49) | None |
| Merson [140] | US, Canada, Netherlands, England | The occurrence between 12 h after arrival to Mexico City and 5 d after departure of any unformed stool not attributed to a preexisting condition plus ≥1 enteric symptom. Or ≥3 watery stools in 24 h | Physicians & their family members | Mainly adults | Mexico | 1/51 (2%) | 1/43 (2.3%) | 0.84 (.01–67.49) | None |
| DuPont [132] | US, Venezuela, Mexico | Acute diarrhea: unformed bowel movements at a daily rate twice of the usual rate plus ≥1 enteric symptom | University clinic | Adult students | Mexico | US 6% (total cases 77), LA 18% (total cases 18) | US 3% (total controls 67), LA 11% (total controls 27) | 2.26 (.43–17.20), 1.60 (.19–13.43) | Country of origin, length of stay in Mexico |
| Bolivar [134] | US, Venezuela, Mexico | Unformed bowel movement at daily rate twice that of the subject's usual rate & ≥1 other enteric symptom | University clinic | Adult students | Mexico | 3/91 (3.3%) | 2/74 (2.7%) | 1.23 (.18–10.54) | Country of origin, length of stay in Mexico |
| Back [127] | Sweden | ≥2 abnormal loose stools/d | Swedish battalion in United Nations forces | Adults | Cyprus | 1/79 (1.3%) | 0/66 (0%) | Serving conditions (next bedfellow) | |
| Echeverria [135] | US | ≥3 loose stools or ≥2 loose stools with other enteric symptom | Soldiers who attended a clinic | Adults | Philippines | 3/152 (2%) | 2/58 (3.5%) | 0.56 (.06–6.94) | None |
| Hogeb [136] | Foreign residents & tourists | Change in normal bowel movements with ≥3 loose stools in 24 h | CIWEC, USEM | All ages | Nepal | 7/148 (4.7%) | 1/95 (1%) | 4.67 (.58–212.52) | Group matching by clinic & season |
| Shlim [128] | Tourists, expatriates | Change in normal bowel movements & ≥3 loose stools in 24 h | CIWEC | ≥18 y | Nepal | 25/189 (13.2%) | 3/112 (2.6%) | 5.54 (1.62–29.23) | None |
| Gascon [137] | Spain | Diarrhea that occurred between 12 h after arriving in & 5 d after departing from the travel country. Diarrhea ≥3 watery stools in 24 h, or unformed stools plus enteric symptom | Tropical Medicine Department | NA | Asia, Africa, Central & Latin America | 11/165 (6.7%) | 3/165 (1.8%) | 3.86 (.99–21.86) | Area visited, controls were relatives or travel companions of cases |
| Schultsz [138] | Netherlands | ≥3 loose stools in 24 h, any number of watery stools in 24 h, or 1–2 loose stools in 24 h plus ≥1 enteric symptom | Outpatient Department for Tropical Diseases | 2–75 y | Asia, Africa, Central & Latin America | Acute 2% (total cases 49), persistent 16.4% (total cases 116) | 4.9% (total controls 102) | 0.40 (.01–3.78), 3.80 (1.30–13.48) | None |
| Boggild [129] | Canada | Diagnosis of giardiasis | Tropical Disease Unit. (GeoSentinel Network) | Mean 37.3 y | International travel | 69/1622 (4.3%) | 5/1906 (0.3%) | 16.9 (6.8–41.9) | None |
| Paschke [130] | Germany | ≥3 unformed stools in 24 h plus ≥1 symptom of enteric infection | Department of Infectious Diseases & Tropical Medicine. | 2–80 y | Asia, Latin America, Europe, other | 7/114 (6.1%) | 3/56 (5.4%) | 1.16 (.25–7.20) | None |
| Pandey [131] | US, Japan, Australia, New Zealand, Western Europe | ≥3 unformed stools in 24 h | CIWEC | >18 y | Nepal | Overall 42/372 (11.3%) ≤7 d 7%, >7 d 26% | 5 (2.9%) | 3.75 (1.40–9.98), 2.48 (.95–7.52), 11.78 (4.41–35.90) | Age, sex, nationality, resident/tourist status, length of stay in Nepal, season |
Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; LA, Latin American (Venezuela and Mexico); CIWEC, Canadian International Water and Energy Consultants; OR, odds ratio; RR, rate ratio; USEM, US Embassy Medical Care.
a ORs and 95% CIs were calculated using the abstracted data from each study. For the study of Andersson et al [139], RR was calculated. Boggild et al [129] reported crude OR and Pandey et al [131] reported adjusted OR.
b Data from the study of Hoge et al [136] were abstracted on 148 cases of diarrhea among which coccidian-like organisms were not identified. Cases and controls from both clinics (CIWEC and USEM) were pooled.
Studies from the 1970s showed a significant association between travelers' diarrhea and Giardia infection among travelers to Leningrad and other sites in the former Soviet Union [133, 139]. Studies of travelers to Nepal revealed that G. lamblia was significantly and strongly associated with diarrhea [128, 131], whereas among travelers to Mexico no significant association was found between G. lamblia and diarrhea [132, 134, 140]. Three other studies of travelers from Canada, Spain, and the Netherlands (whose destinations were Africa, Asia, and Latin America) also showed a significant positive association between G. lamblia and diarrhea or gastrointestinal symptoms [129, 137, 138], and in particular with prolonged (>7 days) and persistent diarrhea (>14 days) [131, 138] (Table 4).
G. LAMBLIA GENOTYPES AND CLINICAL ILLNESS
Experimental challenge studies with G. lamblia in which healthy volunteers were inoculated enterally with trophozoites of 2 distinct human isolates of G. lamblia, designated GS/M and Isr, demonstrated the establishment of infection and elicitation of clinical illness only among participants who were challenged with the GS/M isolate [14], belonging to Giardia assemblage B. This study established the concept that there exists variability among Giardia strains with respect to their pathogenicity for humans [14]. Animal experiments support this concept of variable pathogenicity among Giardia strains [141].
A few recent studies that were undertaken following the availability of techniques to genotype G. lamblia have suggested a possible association between G. lamblia genotypes A or B and clinical illness [63, 80, 105, 142–148] (Table 5). Nevertheless, one must exercise caution in drawing conclusions from these preliminary reports as the sample size of each of these studies was small (6–138 diarrheal cases infected with G. lamblia and 6–199 nondiarrhea subjects infected with G. lamblia). These studies also varied by design, study population, and outcome under investigation (Table 5).
Association Between Giardia lamblia Assemblage and Diarrhea or Other Gastrointestinal Symptoms
| Study & Country . | Design . | Subjects . | Outcome . | No. Cases Genotyped . | No. Controls Genotyped . | Genotype A Cases, No. (%) . | Genotype A Controls, No. (%) . | Genotype B Cases, No. (%) . | Genotype B Controls, No. (%) . | OR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|---|
| Paintlia [147] India | Case series | Adults from gastroenterology & dermatology clinics | Gastrointestinal symptoms: diarrhea, weight loss, abdominal pain | 6 | 6 | 4 (66.7%) | 1 (16.7%) | 2 (33.3%) | 5 (83.3%) | 10.0 (.43–588.32) |
| Eligio-Garcia [63] Mexico | Case series | 6–12 y old children | Chronic/recurrent diarrhea & abdominal pain | 6 | 7 | 6 (100%) | 7 (100%) | 0 (0%) | 0 (0%) | |
| Al-Mohammed [144]b Saudi Arabia | Cross-sectional | Primary school-age children 6–12 y | Acute & chronic diarrhea | 24 | 16 | 7 (29.2%) | 16 (100%) | 15 (62.5%) | 0 (0%) | |
| Molina [146]c Argentina | Cross-sectional | 2–14 y old enrolled at health centers or public schools | Symptoms: diarrhea, anorexia, vomiting, abdominal pain | 50 | 41 | 8 (16%) | 6 (14.6%) | 42 (84%) | 35 (85.4%) | 1.11 (.30–4.28) |
| Aydin [143] Turkey | Case/control | Patients from Dept of Infectious Disease & Gastroenterology | Diarrhea | 20 | 24 | 17 (85%) | 2 (8%) | 3 (15%) | 22 (92%) | 62.33 (9.13–480.26) |
| Sahagun [148]d Spain | Case/control | Ages 2–72 y from outpatient clinic suspected of parasitosis | Symptoms: diarrhea, nausea, abdominal pain/cramps, weight loss, flatulence | 55 | 49 | 29 (52.7%) | 14 (28.5%) | 26 (47.3%) | 35 (71.5%) | 2.79 (1.23–6.38) |
| Haque [105]e Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Hospital controls | Diarrhea | 84 | 199 | 16 (19.5%) | 20 (10.5%) | 68 (80.5%) | 179 (89.5%) | 2.11 (1.04–4.26) |
| Haque [80]f Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Clinic controls | Acute diarrhea | 138 | 184 | 29 (21%) | 10 (5.4%) | 109 (79%) | 174 (94.6%) | 4.63 (2.20–10.27) |
| Read [142] Australia | Longitudinal | Children in day care centers age <5 y | Diarrhea | 9 | 14 | 6 (66.7%) | 1 (7.1%) | 3 (33.3%) | 13 (92.9%) | 26.0 (2.2–304.7) |
| Ajjampur [145]g India | Longitudinal | Newborns followed till age 3 y | Acute & intermediate diarrhea (<14 d) | 45 | 50 | 5 (11.1%) | 2 (4%) | 40 (89.9%) | 48 (96%) | 3.00 (.46–32.74) |
| Study & Country . | Design . | Subjects . | Outcome . | No. Cases Genotyped . | No. Controls Genotyped . | Genotype A Cases, No. (%) . | Genotype A Controls, No. (%) . | Genotype B Cases, No. (%) . | Genotype B Controls, No. (%) . | OR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|---|
| Paintlia [147] India | Case series | Adults from gastroenterology & dermatology clinics | Gastrointestinal symptoms: diarrhea, weight loss, abdominal pain | 6 | 6 | 4 (66.7%) | 1 (16.7%) | 2 (33.3%) | 5 (83.3%) | 10.0 (.43–588.32) |
| Eligio-Garcia [63] Mexico | Case series | 6–12 y old children | Chronic/recurrent diarrhea & abdominal pain | 6 | 7 | 6 (100%) | 7 (100%) | 0 (0%) | 0 (0%) | |
| Al-Mohammed [144]b Saudi Arabia | Cross-sectional | Primary school-age children 6–12 y | Acute & chronic diarrhea | 24 | 16 | 7 (29.2%) | 16 (100%) | 15 (62.5%) | 0 (0%) | |
| Molina [146]c Argentina | Cross-sectional | 2–14 y old enrolled at health centers or public schools | Symptoms: diarrhea, anorexia, vomiting, abdominal pain | 50 | 41 | 8 (16%) | 6 (14.6%) | 42 (84%) | 35 (85.4%) | 1.11 (.30–4.28) |
| Aydin [143] Turkey | Case/control | Patients from Dept of Infectious Disease & Gastroenterology | Diarrhea | 20 | 24 | 17 (85%) | 2 (8%) | 3 (15%) | 22 (92%) | 62.33 (9.13–480.26) |
| Sahagun [148]d Spain | Case/control | Ages 2–72 y from outpatient clinic suspected of parasitosis | Symptoms: diarrhea, nausea, abdominal pain/cramps, weight loss, flatulence | 55 | 49 | 29 (52.7%) | 14 (28.5%) | 26 (47.3%) | 35 (71.5%) | 2.79 (1.23–6.38) |
| Haque [105]e Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Hospital controls | Diarrhea | 84 | 199 | 16 (19.5%) | 20 (10.5%) | 68 (80.5%) | 179 (89.5%) | 2.11 (1.04–4.26) |
| Haque [80]f Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Clinic controls | Acute diarrhea | 138 | 184 | 29 (21%) | 10 (5.4%) | 109 (79%) | 174 (94.6%) | 4.63 (2.20–10.27) |
| Read [142] Australia | Longitudinal | Children in day care centers age <5 y | Diarrhea | 9 | 14 | 6 (66.7%) | 1 (7.1%) | 3 (33.3%) | 13 (92.9%) | 26.0 (2.2–304.7) |
| Ajjampur [145]g India | Longitudinal | Newborns followed till age 3 y | Acute & intermediate diarrhea (<14 d) | 45 | 50 | 5 (11.1%) | 2 (4%) | 40 (89.9%) | 48 (96%) | 3.00 (.46–32.74) |
Abbreviations: CI, confidence interval; ICDDR,B, International Centre for Diarrhoeal Disease Research, Bangladesh; OR, odds ratio.
a The OR presented here reflects the odds of G. lamblia genotype A infection among the cases in comparison to odds of genotype A infection in the control group. The calculations of OR (95% CI) were made using the raw data in the original manuscripts when the authors did not present the measurement of association [80, 143–145, 148].
b Two samples with mixed infections among the cases were not included in the calculation.
c Samples with mixed infections (n = 3) were not included in the calculation. Cases were children with gastrointestinal symptoms.
d Four samples had mixed A and B genotypes, 2 among the symptomatic and 2 among the asymptomatic patients [148]; they were not included in the data presented in this table. Among genotype A isolates, only subgenotype AII was identified [148].
e A total of 267 G. lambia–positive stool specimens were genotyped, among which 16 samples harbored mixed A and B genotypes that were counted twice by the authors, once as A genotype and once as B genotype [105].
fG. lamblia–positive stools of 144 and 199 cases and controls were genotyped; of these 6 and 15 were mixed genotype A and B infections [80], and they were not included in the calculations presented in this table. Part of the G. lamblia genotypes included in this study was reported in an earlier report [105].
g Five mixed infections among the cases and 1 in the control group were excluded from the analysis.
Association Between Giardia lamblia Assemblage and Diarrhea or Other Gastrointestinal Symptoms
| Study & Country . | Design . | Subjects . | Outcome . | No. Cases Genotyped . | No. Controls Genotyped . | Genotype A Cases, No. (%) . | Genotype A Controls, No. (%) . | Genotype B Cases, No. (%) . | Genotype B Controls, No. (%) . | OR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|---|
| Paintlia [147] India | Case series | Adults from gastroenterology & dermatology clinics | Gastrointestinal symptoms: diarrhea, weight loss, abdominal pain | 6 | 6 | 4 (66.7%) | 1 (16.7%) | 2 (33.3%) | 5 (83.3%) | 10.0 (.43–588.32) |
| Eligio-Garcia [63] Mexico | Case series | 6–12 y old children | Chronic/recurrent diarrhea & abdominal pain | 6 | 7 | 6 (100%) | 7 (100%) | 0 (0%) | 0 (0%) | |
| Al-Mohammed [144]b Saudi Arabia | Cross-sectional | Primary school-age children 6–12 y | Acute & chronic diarrhea | 24 | 16 | 7 (29.2%) | 16 (100%) | 15 (62.5%) | 0 (0%) | |
| Molina [146]c Argentina | Cross-sectional | 2–14 y old enrolled at health centers or public schools | Symptoms: diarrhea, anorexia, vomiting, abdominal pain | 50 | 41 | 8 (16%) | 6 (14.6%) | 42 (84%) | 35 (85.4%) | 1.11 (.30–4.28) |
| Aydin [143] Turkey | Case/control | Patients from Dept of Infectious Disease & Gastroenterology | Diarrhea | 20 | 24 | 17 (85%) | 2 (8%) | 3 (15%) | 22 (92%) | 62.33 (9.13–480.26) |
| Sahagun [148]d Spain | Case/control | Ages 2–72 y from outpatient clinic suspected of parasitosis | Symptoms: diarrhea, nausea, abdominal pain/cramps, weight loss, flatulence | 55 | 49 | 29 (52.7%) | 14 (28.5%) | 26 (47.3%) | 35 (71.5%) | 2.79 (1.23–6.38) |
| Haque [105]e Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Hospital controls | Diarrhea | 84 | 199 | 16 (19.5%) | 20 (10.5%) | 68 (80.5%) | 179 (89.5%) | 2.11 (1.04–4.26) |
| Haque [80]f Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Clinic controls | Acute diarrhea | 138 | 184 | 29 (21%) | 10 (5.4%) | 109 (79%) | 174 (94.6%) | 4.63 (2.20–10.27) |
| Read [142] Australia | Longitudinal | Children in day care centers age <5 y | Diarrhea | 9 | 14 | 6 (66.7%) | 1 (7.1%) | 3 (33.3%) | 13 (92.9%) | 26.0 (2.2–304.7) |
| Ajjampur [145]g India | Longitudinal | Newborns followed till age 3 y | Acute & intermediate diarrhea (<14 d) | 45 | 50 | 5 (11.1%) | 2 (4%) | 40 (89.9%) | 48 (96%) | 3.00 (.46–32.74) |
| Study & Country . | Design . | Subjects . | Outcome . | No. Cases Genotyped . | No. Controls Genotyped . | Genotype A Cases, No. (%) . | Genotype A Controls, No. (%) . | Genotype B Cases, No. (%) . | Genotype B Controls, No. (%) . | OR (95% CI)a . |
|---|---|---|---|---|---|---|---|---|---|---|
| Paintlia [147] India | Case series | Adults from gastroenterology & dermatology clinics | Gastrointestinal symptoms: diarrhea, weight loss, abdominal pain | 6 | 6 | 4 (66.7%) | 1 (16.7%) | 2 (33.3%) | 5 (83.3%) | 10.0 (.43–588.32) |
| Eligio-Garcia [63] Mexico | Case series | 6–12 y old children | Chronic/recurrent diarrhea & abdominal pain | 6 | 7 | 6 (100%) | 7 (100%) | 0 (0%) | 0 (0%) | |
| Al-Mohammed [144]b Saudi Arabia | Cross-sectional | Primary school-age children 6–12 y | Acute & chronic diarrhea | 24 | 16 | 7 (29.2%) | 16 (100%) | 15 (62.5%) | 0 (0%) | |
| Molina [146]c Argentina | Cross-sectional | 2–14 y old enrolled at health centers or public schools | Symptoms: diarrhea, anorexia, vomiting, abdominal pain | 50 | 41 | 8 (16%) | 6 (14.6%) | 42 (84%) | 35 (85.4%) | 1.11 (.30–4.28) |
| Aydin [143] Turkey | Case/control | Patients from Dept of Infectious Disease & Gastroenterology | Diarrhea | 20 | 24 | 17 (85%) | 2 (8%) | 3 (15%) | 22 (92%) | 62.33 (9.13–480.26) |
| Sahagun [148]d Spain | Case/control | Ages 2–72 y from outpatient clinic suspected of parasitosis | Symptoms: diarrhea, nausea, abdominal pain/cramps, weight loss, flatulence | 55 | 49 | 29 (52.7%) | 14 (28.5%) | 26 (47.3%) | 35 (71.5%) | 2.79 (1.23–6.38) |
| Haque [105]e Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Hospital controls | Diarrhea | 84 | 199 | 16 (19.5%) | 20 (10.5%) | 68 (80.5%) | 179 (89.5%) | 2.11 (1.04–4.26) |
| Haque [80]f Bangladesh | Matched case/control | All ages, cases from ICDDR,B, Clinic controls | Acute diarrhea | 138 | 184 | 29 (21%) | 10 (5.4%) | 109 (79%) | 174 (94.6%) | 4.63 (2.20–10.27) |
| Read [142] Australia | Longitudinal | Children in day care centers age <5 y | Diarrhea | 9 | 14 | 6 (66.7%) | 1 (7.1%) | 3 (33.3%) | 13 (92.9%) | 26.0 (2.2–304.7) |
| Ajjampur [145]g India | Longitudinal | Newborns followed till age 3 y | Acute & intermediate diarrhea (<14 d) | 45 | 50 | 5 (11.1%) | 2 (4%) | 40 (89.9%) | 48 (96%) | 3.00 (.46–32.74) |
Abbreviations: CI, confidence interval; ICDDR,B, International Centre for Diarrhoeal Disease Research, Bangladesh; OR, odds ratio.
a The OR presented here reflects the odds of G. lamblia genotype A infection among the cases in comparison to odds of genotype A infection in the control group. The calculations of OR (95% CI) were made using the raw data in the original manuscripts when the authors did not present the measurement of association [80, 143–145, 148].
b Two samples with mixed infections among the cases were not included in the calculation.
c Samples with mixed infections (n = 3) were not included in the calculation. Cases were children with gastrointestinal symptoms.
d Four samples had mixed A and B genotypes, 2 among the symptomatic and 2 among the asymptomatic patients [148]; they were not included in the data presented in this table. Among genotype A isolates, only subgenotype AII was identified [148].
e A total of 267 G. lambia–positive stool specimens were genotyped, among which 16 samples harbored mixed A and B genotypes that were counted twice by the authors, once as A genotype and once as B genotype [105].
fG. lamblia–positive stools of 144 and 199 cases and controls were genotyped; of these 6 and 15 were mixed genotype A and B infections [80], and they were not included in the calculations presented in this table. Part of the G. lamblia genotypes included in this study was reported in an earlier report [105].
g Five mixed infections among the cases and 1 in the control group were excluded from the analysis.
The correlation between G. lamblia genotypes and severity of diarrheal illness was examined in an industrialized-country setting among 18 Dutch patients aged 8–60 years with diarrhea and G. lamblia infection who visited their general practitioner [149]. Assemblage A Giardia was found exclusively among the patients with intermittent/mild disease, while all assemblage B Giardia was detected among the patients with more severe cases of diarrhea [149]. Larger studies on the relationship between G. lamblia genotype and diarrhea or other gastrointestinal symptoms mostly showed that genotype B was more common (70%–96% in the controls) than genotype A [80, 105, 143, 145, 146, 148, 150], but a higher detection rate of genotype A was found among the symptomatic patients than the controls. A significant association between genotype A and increased risk of diarrhea or other gastrointestinal symptoms was reported from case/control and longitudinal studies [80, 105, 142, 143, 148]. The relationship between G. lamblia assemblages and diarrhea was examined in a reanalysis [150] of data from a longitudinal study. This study showed no significant difference between G. lamblia genotypes and the number of diarrheal episodes; 0.89 (±0.6) in assemblage A, 1.3 (±1.5) in assemblage B, and 0.80 (±0.84) in mixed infections (P = .58) [150]. A study from Sweden compared the distribution of diarrhea and other gastrointestinal symptoms in patients infected with assemblage A Giardia (n = 51) and subjects infected with assemblage B (n = 87). The reports on diarrhea were similar between the 2 groups (94% and 99% in assemblage A and B, respectively) but flatulence was more common in subjects infected with assemblage B (85%) than A (65%) [151].
DISCUSSION
The confusing, often conflicting, information in the literature on the role of G. lamblia as an enteric pathogen capable of causing diarrheal illness among young children in developing countries led us to undertake this systematic review. Four fundamental conclusions can be drawn from this exercise: (1) G. lamblia is capable of causing both acute and persistent diarrheal illness in adult and pediatric hosts who reside in industrialized countries, including following exposure when they travel to developing countries. (2) G. lamblia does not generally cause acute pediatric diarrhea among infants and children in developing countries, although limited data suggest that infants in the first trimester of life may experience acute clinical diarrhea in response to presumed initial G. lamblia infections. (3) G. lamblia is positively associated with persistent diarrhea among children in developing countries. (4) Genotyping suggests that 2 G. lamblia genotypes (assemblages A and B) may be particularly pathogenic for humans.
Among residents of industrialized countries, evidence from experimental challenge studies of adult volunteers [14, 15], investigations of (particularly water-borne) outbreaks of diarrheal disease [11, 16–18, 23], and investigations of travelers who visit developing countries or known endemic areas [128, 129, 133, 137] collectively and convincingly document that G. lamblia can cause acute diarrheal illness and other gastrointestinal disease. In contrast, as summarized in this review, contradictory results have been reported from epidemiological studies performed in subjects residing in developing countries [24, 72, 82].
Herein we provide the first systematic review and meta-analysis that attempts to address the etiologic role of G. lamblia in relation to diarrheal illness among children from developing countries or other nonindustrialized settings where Giardia is highly endemic. In systematically reviewing the literature, it became apparent that among the many published studies that explored a possible association between G. lamblia and diarrhea, few utilized rigorous design methodology and analytical techniques. For example, few studies controlled for potential confounders and many lacked the statistical power to detect differences between patients with diarrhea and controls without diarrhea. There were very few birth cohort studies, thus the age of first infection could not be assessed. Some studies did not differentiate between the clinical syndromes of acute vs persistent diarrhea, which is critical for analyzing data on Giardia infections; consequently, misclassification of the outcome variable may have ensued. Finally, some studies were limited in duration, covering <1 year.
Accordingly, we limited our analysis to the case/control and cohort studies that utilized rigorous methodology and controlled for potential confounders, lasted ≥ 1 year, and clearly defined the outcome variable (ie, acute vs persistent diarrhea). In so doing, we found there was no significant association between the presence of Giardia in stools and increased risk of acute diarrhea among children living developing countries or nonindustrialized settings [28, 73, 82, 90, 113, 117]. Indeed, there was evidence of a significant inverse association between the presence of Giardia in stools and acute diarrhea among children in developing country or other nonindustrialized settings [68, 70, 72, 80, 86, 98]. A pooled analysis of the studies that utilized rigorous methodology showed that G. lamblia was associated with a 40% lower likelihood of acute diarrhea in children from developing countries (P = .03) (Figure 1).
One may invoke differences in the host, the parasite, or host-parasite interactions to explain the strikingly distinct responses to Giardia exposure among children and adults from industrialized countries vs developing countries. The former are at risk of developing acute diarrhea when they encounter G. lamblia, whereas pediatric subjects in the latter settings experience apparent innocuity or even a protective effect of Giardia against acute diarrhea when infected with this protozoan. One possible explanation may relate to the age of initial exposure and the frequency of subsequent reexposure. In developing-country populations, G. lamblia is ubiquitous and the initial infection is acquired in the first few weeks of life [27, 66, 118, 120, 123, 152, 153]. In developing-country settings, the initial or first few G. lamblia infections may result in diarrhea [74, 123] but immunity is rapidly acquired, thereupon conferring protection against symptomatic disease when subsequently exposed. Giardia lamblia gastroenteritis outbreaks in daycare centers in Canada provide indirect support for this explanation [18, 154]. Children of Canadian origin and those from other industrialized countries were more likely to be infected and to develop Giardia illness compared with children of immigrant families from developing countries [18, 154].
One well-established mechanism by which infants and young children in developing countries are protected against symptomatic disease upon exposure to Giardia is by suckling on mothers whose breast milk contains high titers of anti-Giardia secretory immunoglobulin A (SIgA). Breastfeeding is strongly associated with protection against clinical Giardia diarrhea, even though it does not generally prevent acquisition of G. lamblia infection or chronic carriage [152, 155]. Importantly, clinical protection is correlated with levels of specific anti-G. lamblia SIgA in milk [156]. Analogous evidence derives from experimental challenges of adult US volunteers [14]. Secretory IgA anti-Giardia antibodies were detected in duodenal fluids of subjects who experienced diarrhea following initial challenge with Giardia strain Gsm and these SIgA antibodies correlated with protection against clinical disease when the subjects were rechallenged but not with prevention of reinfection. There are also reports of anti-Giardia properties of breast milk due to moieties other than specific SIgA [157, 158]. Breast milk–derived passive protection may allow the child to acquire active immunity upon exposure to G. lamblia without paying the price of a clinically overt initial infection.
Another possible explanation for the apparent divergent clinical responses to Giardia in industrialized vs developing country pediatric populations may reside in differences in the small intestine. Young children in industrialized countries harbor low numbers of bacteria in their proximal small intestine and their mucosal architecture is characterized by elongated villi and modest numbers of intraepithelial and lamina propria lymphocytes. In contrast, the “normal” small intestine of young children living in impoverished, fecally contaminated conditions in developing countries is marked by blunted villi and hypercellularity of the lamina propria and by small bowel bacterial overgrowth [159–162]. While there is a spectrum of severity of such changes, they are collectively referred to as “environmental enteropathy” (or “tropical enteropathy”) [159–162]. When the small intestine of the young child in the industrialized country setting is exposed to G. lamblia, acute diarrhea or other symptomatology not uncommonly results. In contrast, among young children in developing countries who often manifest environmental enteropathy, G. lamblia appears more often to result in asymptomatic colonization without acute diarrhea. In the environmental enteropathy gut, G. lamblia may modulate the innate immune system and mucosal environment such that a degree of protection is conferred against diarrhea caused by other enteropathogens. In vitro studies show that intestinal mucus may affect Giardia activity [163], and studies in mice suggest that the normal gut flora may play a role in susceptibility to Giardia infection [164]. If this phenomenon is also true in humans, it is possible that these factors might affect the clinical presentation of Giardia infection.
Whereas our systematic review did not find an association between G. lamblia infection and increased risk of acute diarrhea in children in developing-country settings, Giardia was significantly associated with persistent diarrhea in these pediatric populations [106, 107, 109]. The clinical illness of patients with giardiasis in industrialized settings who were infected during outbreaks or during travel to endemic areas also shows that symptoms may persist for several weeks [21, 154, 165, 166].
One must ponder why G. lamblia appears to be associated with a 3-fold increase in the risk of persistent diarrhea among children in developing countries but the pathogen is not associated with an increased risk of acute diarrhea. One hypothesis is that the infants and young children who develop persistent G. lamblia diarrhea constitute a subset of high-risk pediatric hosts because they have more severe chronic undernutrition than their peers of the same age (usually manifest as severe stunting), more severe environmental enteropathy or due to a genetic predisposition (such as combined IgA and immunoglobulin G2 deficiency).
Attributes of the parasite may also account for the propensity to cause persistent diarrhea in children in developing countries. Preliminary evidence supports an association between G. lamblia genotype A and B in the development of clinically overt diarrhea and other gastrointestinal symptoms [80, 105, 142, 143, 148]. Antigenic variation manifested by Giardia may also play a role in the outcome or course of infection. Giardia lamblia trophozoites have variant specific proteins that coat the entire parasite including its flagella. Trophozoites can switch these proteins every 6.5–13.5 generations and this may allow evasion of the immune response, establishment of more persistent infection [36, 167–171], and a propensity to persistent diarrhea.
This systematic review supports the contention that asymptomatic G. lamblia infection somehow protects against diarrheal illness, although mechanistically it is not obvious how this occurs. Giardia lamblia infection triggers both host innate [36–38] and adaptive immune responses [36–38, 172]. Secretion of innate antimicrobial products having anti-Giardia activity (eg, defensin, lactoferrin) by the intestinal epithelium [36–38] and nitric oxide and reactive oxygen species has been described [37, 38]. Secretion of mucins [36, 37] and glycoproteins of the intestinal mucus layer can reduce attachment of a broad range of pathogens to the mucosal surface [36]. These responses elicited by Giardia may negatively affect other pathogens in the gut. Thus, repetitive or prolonged Giardia trophozoite attachment to the intestinal epithelium for extended periods may render the mucosa unfavorable for the attachment of other enteropathogens. Giardia lamblia has also been shown to bind cholera enterotoxin [173] and heavy Giardia muris infection significantly diminishes the intestinal secretion stimulated by cholera toxin compared to mouse intestine without Giardia [174]. Thus, Giardia may offer protection against otherwise severe diarrhea caused by enterotoxigenic bacterial pathogens like Vibrio cholerae and enterotoxigenic E. coli. Finally, one report suggests that the severity of rotavirus gastroenteritis in Bedouin infants may have been significantly reduced in the presence of Giardia lamblia coinfection [29].
Giardia lamblia infection induces serum immunoglobulin M and intestinal SIgA anti-Giardia antibodies [14, 36–38, 172], of which local SIgA is considered the most important for controlling and clearing the infection [37, 38]. Interleukin 6 and T-dependent responses have also been described [36–38]. These anti-Giardia responses may contribute to nonspecific or cross-protection against other enteropathogens [152].
In summary, evidence does not incriminate G. lamblia as a cause of acute diarrhea in young children in developing countries but does suggest an important role of G. lamblia infection in persistent diarrhea in such populations. Statistically well-powered, controlled studies such as the Global Enteric Multicenter Study (GEMS) are needed to clarify the circumstances under which G. lamblia infection may be involved in the development of diarrheal disease. In 7 developing-country sites, GEMS will help address whether Giardia infections in early infancy are positively linked to moderate-to-severe diarrhea, whether some pediatric hosts (eg, more stunted) are more prone to develop persistent diarrhea, whether Giardia decreases the risk of acute diarrhea from other specific enteropathogens, and whether specific Giardia genotypes exhibit enhanced pathogenicity over other genotypes.
Notes
Financial support. This study was funded by the Bill & Melinda Gates Foundation (grant number 38874).
Supplement sponsorship. This article was published as part of the supplement entitled “The Global Enteric Multicenter Study (GEMS),” sponsored by the Bill & Melinda Gates Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




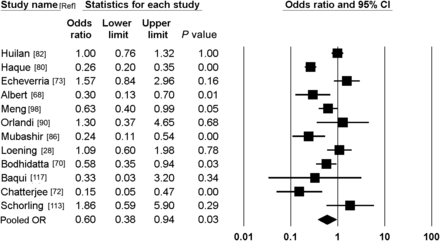
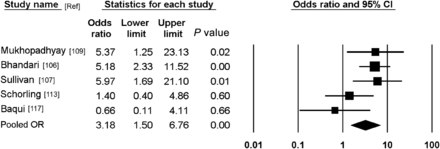
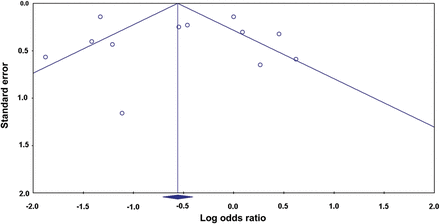
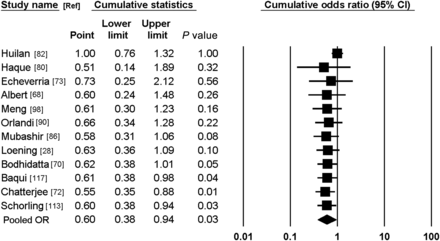

Comments