-
PDF
- Split View
-
Views
-
Cite
Cite
Karl Swedberg, Lars G. Olsson, Andrew Charlesworth, John Cleland, Peter Hanrath, Michel Komajda, Marco Metra, Christian Torp-Pedersen, Philip Poole-Wilson, Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET, European Heart Journal, Volume 26, Issue 13, July 2005, Pages 1303–1308, https://doi.org/10.1093/eurheartj/ehi166
Close - Share Icon Share
Abstract
Aims Atrial fibrillation is common in patients with chronic heart failure (CHF). We analysed the risk associated with atrial fibrillation in a large cohort of patients with chronic heart failure all treated with a beta-blocker.
Methods and results In COMET, 3029 patients with CHF were randomized to carvedilol or metoprolol tartrate and followed for a mean of 58 months. We analysed the prognostic relevance on other outcomes of atrial fibrillation on the baseline electrocardiogram compared with no atrial fibrillation and the impact of new onset atrial fibrillation during follow-up. A multivariate analysis was performed using a Cox regression model where 10 baseline covariates were entered together with study treatment allocation. Six hundred patients (19.8%) had atrial fibrillation at baseline. These patients were older (65 vs. 61 years), included more men (88 vs.78%), had more severe symptoms [higher New York Heart Association (NYHA) class] and a longer duration of heart failure (all P<0.0001). Atrial fibrillation was associated with significantly increased mortality [relative risk (RR) 1.29: 95% CI 1.12–1.48; P<0.0001], higher all-cause death or hospitalization (RR 1.25: CI 1.13–1.38), and cardiovascular death or hospitalization for worsening heart failure (RR 1.34: CI 1.20–1.52), both P<0.0001. By multivariable analysis, atrial fibrillation no longer independently predicted mortality. Beneficial effects on mortality by carvedilol remained significant (RR 0.836: CI 0.74–0.94; P=0.0042). New onset atrial fibrillation during follow-up (n=580) was associated with significant increased risk for subsequent death in a time-dependent analysis (RR 1.90: CI 1.54–2.35; P<0.0001) regardless of treatment allocation and changes in NYHA class.
Conclusion In CHF, atrial fibrillation significantly increases the risk for death and heart failure hospitalization, but is not an independent risk factor for mortality after adjusting for other predictors of prognosis. Treatment with carvedilol compared with metoprolol offers additional benefits among patients with atrial fibrillation. Onset of new atrial fibrillation in patients on long-term beta-blocker therapy is associated with significant increased subsequent risk of mortality and morbidity.
Introduction
Atrial fibrillation is a common condition in patients with chronic heart failure (CHF) and left ventricular dysfunction and it is the most prevalent sustained arrhythmia among these patients. The prevalence of atrial fibrillation in large clinical trials varies between 13 and 50%, depending on the severity of heart failure.1–11 Population-based studies have found the incidence and prevalence to increase with age and severity of heart failure.12
A recent report from the Euroheart Failure survey found that 23% of 11 327 patients with heart failure had chronic atrial fibrillation.13 The haemodynamic consequences of atrial fibrillation include inappropriate ventricular rate, loss of atrial contraction, and elevated filling pressures causing atrial dilatation and reductions in stroke volume. There is no consensus as to whether atrial fibrillation is an independent risk factor for morbidity and mortality in heart failure or just a marker of more advanced disease. The prognostic importance of atrial fibrillation has been assessed in several large studies2,5,14–17 and some outpatient groups with severe heart failure18–20 but the findings have been inconsistent.
In the Carvedilol or Metoprolol European Trial (COMET), the effect of carvedilol compared with metoprolol tartrate was evaluated in patients with CHF during a mean follow-up of 58 months.21 In the present report, we have analysed the prognostic relevance of atrial fibrillation at baseline as well the importance of new-onset atrial fibrillation on subsequent patient outcome.
Methods
COMET was a randomized, double-blind comparison of carvedilol with metoprolol tartrate. A detailed description of study design and inclusion/exclusion criteria has been published earlier.22 In summary, eligible patients had symptomatic CHF [New York Heart Association (NYHA) class II–IV] and at least one cardiovascular admission during the previous 2 years. Left-ventricular ejection fraction had to be ≤0.35 measured within the previous 3 months by echocardiography or radionuclide ventriculography. Major exclusion criteria were requirement for intravenous inotropic therapy, current treatment with calcium channel blockers, amiodarone (>200 mg/day), class I anti-arrhythmic drugs, or administration of any investigational drug within the preceding 30 days, unstable angina, myocardial infarction, coronary revascularization or stroke within the previous 2 months, uncontrolled hypertension, haemodynamically significant valvular disease, or symptomatic ventricular arrhythmias within the past 2 months not adequately treated with anti-arrhythmic drugs.
At randomization, 3029 patients were allocated to receive either 3.125 mg carvedilol twice daily, 5 mg metoprolol tartrate twice daily with dose doubling bi-weekly until maximally tolerated or a target dose of carvedilol 25 mg twice daily or metoprolol 50 mg twice daily was achieved. Patient demographics were obtained at randomization together with a 12-lead electrocardiogram (ECG) and further baseline assessments. Clinical follow-up investigations were performed in 1 year intervals including a 12-lead ECG.
Analysis
On the basis of the presence of atrial fibrillation on the baseline ECG, patients were grouped as No AF or AF. Patients with a history of atrial fibrillation with sinus rhythm at baseline ECG were considered to have paroxysmal atrial fibrillation and included in the No AF group. Patients with sinus rhythm at baseline and ECG documented atrial fibrillation during follow-up were classified as new onset AF.
The primary outcome of COMET was all-cause mortality. A co-primary outcome was all-cause mortality or all-cause hospital admission. Secondary outcomes included cardiovascular death, worsening heart failure, or the composite cardiovascular death or hospitalization for worsening heart failure. An endpoint committee consisting of three experienced cardiologists classified death as cardiovascular or non-cardiovascular.
Statistical analysis
Differences between patients with or without atrial fibrillation were made using χ2 tests for categorical data and t-tests for continuous parameters. Kaplan–Meier estimates for mortality were calculated and differences between the groups assessed using Cox proportional hazard models. In order to adjust for all significant prognostic factors that might affect outcome, we produced a multivariable Cox regression model using baseline variables presumed to be of prognostic importance: age, gender, ejection fraction, blood pressure, NYHA class, aetiology, previous angina, S-creatinine, S-sodium, and dose of furosemide. Decisions regarding whether to include continuous parameters as linear covariates or as multi category factors were based on the functional form of each variable as a predictor obtained from Martingale residual plots. Where the plot did not appear to be linear, and there was no appropriate transformation, cut points were chosen from the plot to create categorical variables. The prognostic significance of new onset AF was assessed using a time-dependent Cox regression analysis. The same sets of baseline variables were included, and new onset AF and the NYHA class were introduced as time-dependent covariates. It is noted that the results presented were identical when adjustment was made for all significant baseline predictors, obtained for each endpoint using forward and backward stepwise procedures (data not shown).
All tests performed were two-sided and the significance level was 0.05. No attempt has been made to adjust the significance level of the data presented for multiple testing.
Results
There were 600 patients (19.8%) who presented with atrial fibrillation at the baseline ECG. Baseline demographic variables by the presence of atrial fibrillation are presented in Table 1. Patients with atrial fibrillation were older (65 vs. 61 years; P<0.0001), more often males (88 vs. 78%; P<0.0001), had more severe heart failure symptoms as reflected by NYHA class, and had a longer duration of CHF. Ischaemic heart disease and dilated cardiomyopathy were the two most common aetiologies of CHF in both groups although the former was less common in the AF group (43 vs. 55%; P<0.0001). Accordingly aspirin and, although not frequently used, lipid lowering therapy were more common in patients with sinus rhythm, whereas baseline atrial fibrillation was more often associated with treatment with digitalis, anti-arrhythmics, and anticoagulants.
Outcomes
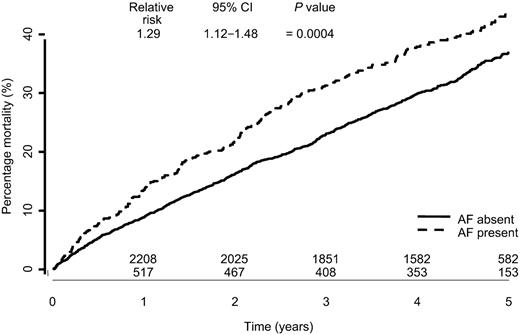
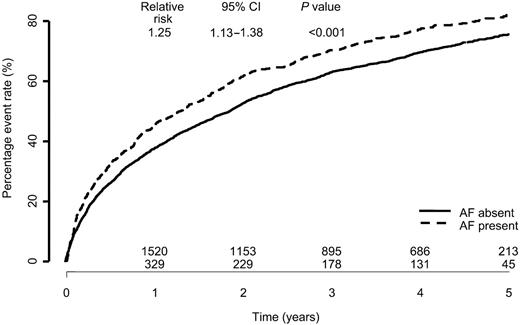
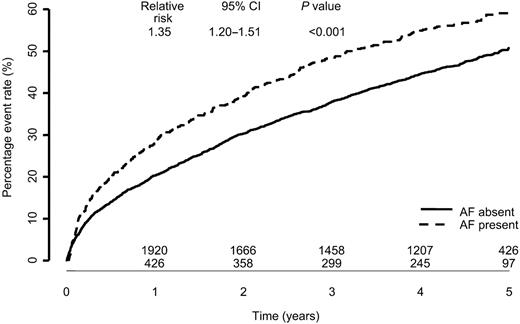
The presence of atrial fibrillation at baseline ECG compared with no atrial fibrillation was associated with significantly increased all-cause mortality over a 5 year follow-up period [relative risk (RR) 1.29: 95% CI 1.12–1.48; P=0.0004, Figure 1]. Patients with atrial fibrillation also experienced a higher all-cause death or all-cause hospitalization rate (RR 1.25: CI 1.13–1.38) as well as cardiovascular death and hospitalizations for worsening heart failure rate (RR 1.35: CI 1.20–1.52; both P<0.001) (Figures 2 and 3).
A total of 11 pre-specified patient variables obtained at baseline including allocation group were included in a post hoc regression analysis model. After adjustment for baseline covariates in the Cox regression analysis, presence of atrial fibrillation was no longer significantly associated with all-cause mortality (Table 2). However, for all-cause mortality or all-cause hospitalizations, atrial fibrillation had independent prognostic impact (RR 1.13: CI 1.02–1.26; P=0.025). Furthermore, atrial fibrillation was of independent significant importance for all-cause mortality or hospitalization for worsening heart failure (RR 1.19: CI 1.05–1.35; P=0.007).
After adjustment for age and gender only, atrial fibrillation was no longer of independent prognostic importance for mortality. Allocation to carvedilol therapy remained of independent beneficial importance for all-cause mortality in this model (RR 0.836: CI 0.74–0.94; P=0.0042).
Risk after new onset AF during follow-up
In 580 of 2429 patients with sinus rhythm at baseline, onset of atrial fibrillation were reported during the study. New onset atrial fibrillation remained an independent predictor of subsequent all-cause mortality when treated as a time-dependent variable (RR 1.90: CI 1.54–2.35; P<0.0001) regardless of treatment allocation and changes in NYHA classification over time (Figure 4 and Table 3). Treatment allocation to carvedilol or metoprolol did not affect incidence of atrial fibrillation (RR 0.93; P=0.2).
Discussion
In this analysis of COMET including 3029 patients treated with a beta-blocker during a follow-up of 58 months, the presence of atrial fibrillation at baseline was associated with a 28% increased risk of death. However, when adjusted for 10 baseline covariates plus allocation group, this prognostic impact was lost for mortality, but retained as regards the composite of mortality or all-cause hospitalizations as well as hospitalizations for worsening heart failure. The major differences between the two groups in age, gender, NYHA classification, and previous myocardial infarction were probably more important prognostic factors than atrial fibrillation. Our findings also demonstrate the beneficial long-term effects of treatment with carvedilol in these patients, as allocation to carvedilol compared with metoprolol remained significant in the multivariable analysis.
Our findings contrast with several earlier reports. The major difference with our study and previous reports is the long-term use of beta-blockers in COMET. The multivariable model included many markers of the severity of heart failure, some of which may be present in patients both with and without atrial fibrillation.
Middlekauff et al.23 evaluated the relationship of atrial fibrillation to overall survival and sudden death in 390 consecutive patients with advanced heart failure. They showed that 1 year survival rate was significantly worse for patients with atrial fibrillation than for patients with sinus rhythm (52 vs. 71%; P=0.001). Atrial fibrillation was independently associated with an increased risk of sudden death. Carson et al.2 reported from the Vasodilator in Heart Failure Trials (V-HeFT) and suggested that atrial fibrillation did not increase morbidity or mortality. This study included only patients with mild to moderate CHF, and patients with atrial fibrillation enrolled in V-HeFT II had significantly higher mean left ventricular ejection fractions than those without atrial fibrillation. Importantly, in both V-Heft studies, the investigators decided the treatment for atrial fibrillation. Stevenson et al.24 reported the prognostic importance of atrial fibrillation in patients with severe heart failure during the period from 1985 to 1989 and during the period from 1990 to 1993. In the first period, atrial fibrillation was associated with a worse outcome, which had disappeared in the second period. They suggested that the difference in mortality was due to a change in medication. Use of class I anti-arrhythmic agents had decreased and treatment with ACE-inhibitors had been introduced. In a retrospective analysis of the SOLVD study, baseline atrial fibrillation was an independent predictor of all-cause mortality (RR 1.34: 95% CI 1.12–1.62; P=0.002), progressive pump-failure death, combined endpoint of death, or hospitalization for heart failure but not for arrhythmic death.5 Mahoney et al.20 studied 234 patients eligible for heart transplantation. Mortality was similar in those with atrial fibrillation as with sinus rhythm. Atrial fibrillation was not an independent predictor of mortality. Crijns et al.15 examined 409 patients with moderate to severe CHF from the PRIME II study and compared patients with sinus rhythm (n=325) to those with atrial fibrillation (n=84). Overall mortality was higher among patients with atrial fibrillation (60%) compared with those with sinus rhythm (47%; P=0.04) but the difference did not remain when adjusted for baseline covariates, similar to our findings. During the 3.4 year follow-up, 30 of 325 patients with sinus rhythm developed atrial fibrillation. The development of atrial fibrillation was not an independent predictor of subsequent all-cause mortality but the power in this analysis is limited. In the DIG trial, 866 patients (11.1%) developed supraventricular tachycardia at least once during 37 months of follow-up.16 This event was associated with a greater risk of mortality (RR 2.453; P=0.0001) and stroke (RR 2.379; P=0.0001).
Two trials have evaluated prognostic value of conversion to sinus rhythm. In CHF-STAT atrial fibrillation substudy, amiodarone reduced mortality only if the patients converted to sinus rhythm (16 converters, 35 non-converters, P=0.04).14 During a 4 year follow-up, 33 out of 531 patients developed atrial fibrillation.
In 506 patients enrolled in DIAMOND CHF and post-MI studies, conversion to sinus rhythm was associated with a reduced subsequent all-cause mortality (RR 0.44: 95% CI 0.30–0.64; P<0.0001) with similar results regardless of treatment allocation and mechanism of conversion.17 In the CHF study, 46 of 1080 patients with sinus rhythm at baseline developed atrial fibrillation during the 18 month follow-up.7
Anti-arrhythmic therapy at baseline was associated with a significantly independent increased risk. By design, treatment with a class I anti-arrhythmic agent or amiodarone >200 mg/day was an exclusion criteria in COMET. Thus, the anti-arrhythmic group was almost entirely composed of patients on amiodarone. Amiodarone has been shown to improve survival in CHF in a randomized open trial3 or not to increase risk in CHF-STAT.4 Dofetilide, a class III agent, has been shown not to have a neutral effect on mortality in CHF patients.7 Together with beta-blockers, amiodarone was found to have beneficial effects on mortality and morbidity in post-MI patients in EMIAT and CAMIAT.25 However, in CIBIS-II, treatment with digitalis or amiodarone was independently associated with hospitalization for CHF with a borderline significant relationship with mortality for amiodarone.10 In the DIG trial, there were more deaths reported in the digoxin group because of tachy- and brady-arrhythmias.26
The adverse prognosis associated with agents as well as pacemaker treatment in our analysis, may be due to imbalance in the risk-profile among patients. However, the divergent findings between earlier trials and pivotal beta-blocker trials warrant further analyses. The ongoing AF–CHF trial will probably shed some light in this context.27
Statin therapy at baseline was associated with a significantly lower risk. However, similar observations have been made in other studies and this observation probably signals different management and risk profiles not fully reflected in baseline demographics.28
Beta-blockers in CHF and atrial fibrillation
There has been no prospective trial on the effects of beta-blockers on outcomes in patients with CHF and atrial fibrillation. However, there have been reports from subgroups of trials. In the US carvedilol heart failure study, left ventricular ejection fraction and patients global assessment improved in a manner similar to the overall study.9 The authors concluded that carvedilol probably reduces the combined endpoint of all-cause mortality and CHF hospitalization (7 vs. 19%; P=0.055 in study) in patients with atrial fibrillation. In CIBIS-II, the beneficial effect on survival by bisoprolol was observed in patients with sinus rhythm but not among those with atrial fibrillation (RR 1.161; P=0.55 for atrial fibrillation patients vs. RR 0.577; P=0.0003 for sinus rhythm patients).10
In our analysis, we included only patients who were treated with a beta-blocker by design in COMET. Any beneficial effect by beta-blocker treatment was accordingly already taken into account. In this context, the presence of atrial fibrillation did not remain an independent prognostic factor for important clinical outcomes in the multivariable Cox regression model. Other prognostically important covariates became more significant, for example, age, gender, NYHA classification, ejection fraction, aetiology of CHF, or concomitant diseases. Furthermore, randomization to carvedilol treatment remained of independent importance in this model. It is interesting that during follow-up, incidence of new atrial fibrillation was associated with significant increased risk of subsequent mortality. Also, the incidence of new onset AF was high. Thus, even if atrial fibrillation was not an independent prognostic factor at baseline, the development of atrial fibrillation in patients with CHF during long-term treatment with beta-blockers is associated with significant subsequent adverse outcome including increased mortality.
Limitations
This study was not designed to prospectively assess the importance of atrial fibrillation. The presence of atrial fibrillation at the baseline ECG was used as definition of atrial fibrillation. We can therefore not evaluate the importance of paroxysmal compared with sustained atrial fibrillation. Incidence of new atrial fibrillation was also assessed by adverse events reporting or presence at ECG at the final visit, which of course was only possible in survivors. The impact of atrial fibrillation is therefore probably underestimated in this analysis. However, the long-term follow-up of 5 years and the large number of events, 600 patients at baseline and another 580 patients during follow-up, importantly contributes to our evaluation and makes it clinically meaningful.
Conclusion
In patients with CHF, the presence of atrial fibrillation and new onset of atrial fibrillation are common complications and have important clinical implications. Experience from COMET demonstrates additional benefits on mortality and morbidity of carvedilol in relation to metoprolol tartrate in these patients.
Figure 1 All-cause mortality by baseline atrial fibrillation.
Figure 2 All-cause mortality or all-cause hospitalizations by baseline atrial fibrillation.
Figure 3 Cardiovascular mortality or hospitalization for worsening heart failure by baseline atrial fibrillation.
Figure 4 Mortality following new onset AF in patients with sinusrythm at baseline.
Baseline characteristics by the presence of atrial fibrillation
| . | AF at baseline (n=600) . | No AF at baseline (n=2429) . | Total (n=3029) . | P-value . |
|---|---|---|---|---|
| Age (years) mean/SD | 65.1/10.0 | 61.2/11.6 | 62.0/11.4 | <0.0001 |
| Gender (% male) | 87.5 | 77.9 | 79.8 | <0.0001 |
| Race (% white) | 99.7 | 98.8 | 99.0 | 0.3 |
| Body mass index (kg/m2) mean | 27.5 | 26.7 | 26.9 | 0.0001 |
| Systolic BP (mmHg) mean | 127.3 | 125.8 | 126.1 | 0.085 |
| Diastolic BP (mmHg) mean | 78.0 | 76.9 | 77.1 | 0.037 |
| Heart rate (bpm) mean | 82.5 | 80.8 | 81.1 | 0.005 |
| NYHA class | ||||
| % II | 37.8 | 51.0 | 48.4 | <0.0001 |
| % III | 57.5 | 45.4 | 47.8 | |
| % IV | 4.7 | 3.6 | 3.8 | |
| Duration CHF (months) mean/median | 55.3/33.0 | 39.2/18.0 | 42.4/21.0 | <0.0001 |
| Aetiology CHFa | ||||
| % Ischaemic heart disease | 43.0 | 54.9 | 52.5 | <0.0001 |
| % Hypertension | 22.3 | 16.6 | 17.7 | 0.0010 |
| Ejection fraction (%) mean | 26.2 | 26.1 | 26.1 | 0.719 |
| Previous MI (%) | 31.1 | 44.1 | 41.5 | <0.0001 |
| CAD (confirmed by angiography) (%) | 52.8 | 60.1 | 58.9 | 0.016 |
| Current angina (%) | 20.6 | 21.9 | 21.6 | 0.494 |
| Hypertension (%) | 39.9 | 36.1 | 36.9 | 0.088 |
| Diabetes (%) | 24.8 | 24.0 | 24.2 | 0.675 |
| Stroke (%) | 8.6 | 6.7 | 7.1 | 0.112 |
| ECG findings at baselinea | ||||
| % Sinus rhythm | 0.8 | 92.8 | 74.6 | <0.0001 |
| % Atrial fibrillation/flutter | 100.0 | 0.0 | 19.8 | |
| % Paced rhythm | 5.2 | 6.9 | 6.5 | 0.13 |
| % LBBB | 3.7 | 6.0 | 5.5 | 0.027 |
| Concomitant medication at randomization | ||||
| Diureticsb (%) | 99.0 | 98.6 | 98.7 | 0.485 |
| ACE‐inhibitors (%) | 92.2 | 91.1 | 91.4 | 0.427 |
| Angiotensin receptor antagonists (%) | 6.2 | 6.6 | 6.5 | 0.682 |
| Digitalis (%) | 81.2 | 54.1 | 59.4 | <0.0001 |
| Anti-arrhythmics (%) | 16.3 | 11.1 | 12.1 | 0.0005 |
| Nitrates (%) | 29.2 | 33.6 | 32.8 | 0.037 |
| Aldosterone antagonists (%) | 12.2 | 10.5 | 10.8 | 0.227 |
| Beta-blockersc (%) | 4.7 | 4.2 | 4.3 | 0.613 |
| Anticoagulants (%) | 74.5 | 38.6 | 45.7 | <0.0001 |
| Aspirin (%) | 19.0 | 41.3 | 36.8 | <0.0001 |
| Lipid lowering agents (statins) (%) | 10.5 | 23.7 | 21.1 | <0.0001 |
| . | AF at baseline (n=600) . | No AF at baseline (n=2429) . | Total (n=3029) . | P-value . |
|---|---|---|---|---|
| Age (years) mean/SD | 65.1/10.0 | 61.2/11.6 | 62.0/11.4 | <0.0001 |
| Gender (% male) | 87.5 | 77.9 | 79.8 | <0.0001 |
| Race (% white) | 99.7 | 98.8 | 99.0 | 0.3 |
| Body mass index (kg/m2) mean | 27.5 | 26.7 | 26.9 | 0.0001 |
| Systolic BP (mmHg) mean | 127.3 | 125.8 | 126.1 | 0.085 |
| Diastolic BP (mmHg) mean | 78.0 | 76.9 | 77.1 | 0.037 |
| Heart rate (bpm) mean | 82.5 | 80.8 | 81.1 | 0.005 |
| NYHA class | ||||
| % II | 37.8 | 51.0 | 48.4 | <0.0001 |
| % III | 57.5 | 45.4 | 47.8 | |
| % IV | 4.7 | 3.6 | 3.8 | |
| Duration CHF (months) mean/median | 55.3/33.0 | 39.2/18.0 | 42.4/21.0 | <0.0001 |
| Aetiology CHFa | ||||
| % Ischaemic heart disease | 43.0 | 54.9 | 52.5 | <0.0001 |
| % Hypertension | 22.3 | 16.6 | 17.7 | 0.0010 |
| Ejection fraction (%) mean | 26.2 | 26.1 | 26.1 | 0.719 |
| Previous MI (%) | 31.1 | 44.1 | 41.5 | <0.0001 |
| CAD (confirmed by angiography) (%) | 52.8 | 60.1 | 58.9 | 0.016 |
| Current angina (%) | 20.6 | 21.9 | 21.6 | 0.494 |
| Hypertension (%) | 39.9 | 36.1 | 36.9 | 0.088 |
| Diabetes (%) | 24.8 | 24.0 | 24.2 | 0.675 |
| Stroke (%) | 8.6 | 6.7 | 7.1 | 0.112 |
| ECG findings at baselinea | ||||
| % Sinus rhythm | 0.8 | 92.8 | 74.6 | <0.0001 |
| % Atrial fibrillation/flutter | 100.0 | 0.0 | 19.8 | |
| % Paced rhythm | 5.2 | 6.9 | 6.5 | 0.13 |
| % LBBB | 3.7 | 6.0 | 5.5 | 0.027 |
| Concomitant medication at randomization | ||||
| Diureticsb (%) | 99.0 | 98.6 | 98.7 | 0.485 |
| ACE‐inhibitors (%) | 92.2 | 91.1 | 91.4 | 0.427 |
| Angiotensin receptor antagonists (%) | 6.2 | 6.6 | 6.5 | 0.682 |
| Digitalis (%) | 81.2 | 54.1 | 59.4 | <0.0001 |
| Anti-arrhythmics (%) | 16.3 | 11.1 | 12.1 | 0.0005 |
| Nitrates (%) | 29.2 | 33.6 | 32.8 | 0.037 |
| Aldosterone antagonists (%) | 12.2 | 10.5 | 10.8 | 0.227 |
| Beta-blockersc (%) | 4.7 | 4.2 | 4.3 | 0.613 |
| Anticoagulants (%) | 74.5 | 38.6 | 45.7 | <0.0001 |
| Aspirin (%) | 19.0 | 41.3 | 36.8 | <0.0001 |
| Lipid lowering agents (statins) (%) | 10.5 | 23.7 | 21.1 | <0.0001 |
MI, myocardial infarction; CAD, coronary artery disease.
aMore than one answer possible.
bInclusion criteria.
cStopped prior to study start.
Baseline characteristics by the presence of atrial fibrillation
| . | AF at baseline (n=600) . | No AF at baseline (n=2429) . | Total (n=3029) . | P-value . |
|---|---|---|---|---|
| Age (years) mean/SD | 65.1/10.0 | 61.2/11.6 | 62.0/11.4 | <0.0001 |
| Gender (% male) | 87.5 | 77.9 | 79.8 | <0.0001 |
| Race (% white) | 99.7 | 98.8 | 99.0 | 0.3 |
| Body mass index (kg/m2) mean | 27.5 | 26.7 | 26.9 | 0.0001 |
| Systolic BP (mmHg) mean | 127.3 | 125.8 | 126.1 | 0.085 |
| Diastolic BP (mmHg) mean | 78.0 | 76.9 | 77.1 | 0.037 |
| Heart rate (bpm) mean | 82.5 | 80.8 | 81.1 | 0.005 |
| NYHA class | ||||
| % II | 37.8 | 51.0 | 48.4 | <0.0001 |
| % III | 57.5 | 45.4 | 47.8 | |
| % IV | 4.7 | 3.6 | 3.8 | |
| Duration CHF (months) mean/median | 55.3/33.0 | 39.2/18.0 | 42.4/21.0 | <0.0001 |
| Aetiology CHFa | ||||
| % Ischaemic heart disease | 43.0 | 54.9 | 52.5 | <0.0001 |
| % Hypertension | 22.3 | 16.6 | 17.7 | 0.0010 |
| Ejection fraction (%) mean | 26.2 | 26.1 | 26.1 | 0.719 |
| Previous MI (%) | 31.1 | 44.1 | 41.5 | <0.0001 |
| CAD (confirmed by angiography) (%) | 52.8 | 60.1 | 58.9 | 0.016 |
| Current angina (%) | 20.6 | 21.9 | 21.6 | 0.494 |
| Hypertension (%) | 39.9 | 36.1 | 36.9 | 0.088 |
| Diabetes (%) | 24.8 | 24.0 | 24.2 | 0.675 |
| Stroke (%) | 8.6 | 6.7 | 7.1 | 0.112 |
| ECG findings at baselinea | ||||
| % Sinus rhythm | 0.8 | 92.8 | 74.6 | <0.0001 |
| % Atrial fibrillation/flutter | 100.0 | 0.0 | 19.8 | |
| % Paced rhythm | 5.2 | 6.9 | 6.5 | 0.13 |
| % LBBB | 3.7 | 6.0 | 5.5 | 0.027 |
| Concomitant medication at randomization | ||||
| Diureticsb (%) | 99.0 | 98.6 | 98.7 | 0.485 |
| ACE‐inhibitors (%) | 92.2 | 91.1 | 91.4 | 0.427 |
| Angiotensin receptor antagonists (%) | 6.2 | 6.6 | 6.5 | 0.682 |
| Digitalis (%) | 81.2 | 54.1 | 59.4 | <0.0001 |
| Anti-arrhythmics (%) | 16.3 | 11.1 | 12.1 | 0.0005 |
| Nitrates (%) | 29.2 | 33.6 | 32.8 | 0.037 |
| Aldosterone antagonists (%) | 12.2 | 10.5 | 10.8 | 0.227 |
| Beta-blockersc (%) | 4.7 | 4.2 | 4.3 | 0.613 |
| Anticoagulants (%) | 74.5 | 38.6 | 45.7 | <0.0001 |
| Aspirin (%) | 19.0 | 41.3 | 36.8 | <0.0001 |
| Lipid lowering agents (statins) (%) | 10.5 | 23.7 | 21.1 | <0.0001 |
| . | AF at baseline (n=600) . | No AF at baseline (n=2429) . | Total (n=3029) . | P-value . |
|---|---|---|---|---|
| Age (years) mean/SD | 65.1/10.0 | 61.2/11.6 | 62.0/11.4 | <0.0001 |
| Gender (% male) | 87.5 | 77.9 | 79.8 | <0.0001 |
| Race (% white) | 99.7 | 98.8 | 99.0 | 0.3 |
| Body mass index (kg/m2) mean | 27.5 | 26.7 | 26.9 | 0.0001 |
| Systolic BP (mmHg) mean | 127.3 | 125.8 | 126.1 | 0.085 |
| Diastolic BP (mmHg) mean | 78.0 | 76.9 | 77.1 | 0.037 |
| Heart rate (bpm) mean | 82.5 | 80.8 | 81.1 | 0.005 |
| NYHA class | ||||
| % II | 37.8 | 51.0 | 48.4 | <0.0001 |
| % III | 57.5 | 45.4 | 47.8 | |
| % IV | 4.7 | 3.6 | 3.8 | |
| Duration CHF (months) mean/median | 55.3/33.0 | 39.2/18.0 | 42.4/21.0 | <0.0001 |
| Aetiology CHFa | ||||
| % Ischaemic heart disease | 43.0 | 54.9 | 52.5 | <0.0001 |
| % Hypertension | 22.3 | 16.6 | 17.7 | 0.0010 |
| Ejection fraction (%) mean | 26.2 | 26.1 | 26.1 | 0.719 |
| Previous MI (%) | 31.1 | 44.1 | 41.5 | <0.0001 |
| CAD (confirmed by angiography) (%) | 52.8 | 60.1 | 58.9 | 0.016 |
| Current angina (%) | 20.6 | 21.9 | 21.6 | 0.494 |
| Hypertension (%) | 39.9 | 36.1 | 36.9 | 0.088 |
| Diabetes (%) | 24.8 | 24.0 | 24.2 | 0.675 |
| Stroke (%) | 8.6 | 6.7 | 7.1 | 0.112 |
| ECG findings at baselinea | ||||
| % Sinus rhythm | 0.8 | 92.8 | 74.6 | <0.0001 |
| % Atrial fibrillation/flutter | 100.0 | 0.0 | 19.8 | |
| % Paced rhythm | 5.2 | 6.9 | 6.5 | 0.13 |
| % LBBB | 3.7 | 6.0 | 5.5 | 0.027 |
| Concomitant medication at randomization | ||||
| Diureticsb (%) | 99.0 | 98.6 | 98.7 | 0.485 |
| ACE‐inhibitors (%) | 92.2 | 91.1 | 91.4 | 0.427 |
| Angiotensin receptor antagonists (%) | 6.2 | 6.6 | 6.5 | 0.682 |
| Digitalis (%) | 81.2 | 54.1 | 59.4 | <0.0001 |
| Anti-arrhythmics (%) | 16.3 | 11.1 | 12.1 | 0.0005 |
| Nitrates (%) | 29.2 | 33.6 | 32.8 | 0.037 |
| Aldosterone antagonists (%) | 12.2 | 10.5 | 10.8 | 0.227 |
| Beta-blockersc (%) | 4.7 | 4.2 | 4.3 | 0.613 |
| Anticoagulants (%) | 74.5 | 38.6 | 45.7 | <0.0001 |
| Aspirin (%) | 19.0 | 41.3 | 36.8 | <0.0001 |
| Lipid lowering agents (statins) (%) | 10.5 | 23.7 | 21.1 | <0.0001 |
MI, myocardial infarction; CAD, coronary artery disease.
aMore than one answer possible.
bInclusion criteria.
cStopped prior to study start.
Multivariable analysis of risk of all-cause mortality in patients with AF vs. No AF at baseline
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.836 | 0.74, 0.945 | 0.0042 |
| Increasing age | 1.036 | 1.029, 1.043 | <0.001 |
| Female vs. male | 0.868 | 0.738, 1.02 | 0.0855 |
| Increasing systolic BP | 0.992 | 0.988, 0.995 | <0.001 |
| Increasing LVEF | 0.98 | 0.971, 0.988 | <0.001 |
| IHD vs. rest | 1.326 | 1.154, 1.522 | 0.0001 |
| NYHA III vs. NYHA II | 1.439 | 1.259, 1.645 | <0.001 |
| NYHA IV vs. NYHA II | 1.827 | 1.392, 2.398 | <0.001 |
| Previous angina | 0.939 | 0.809, 1.09 | 0.4078 |
| Increasing sodium | 0.941 | 0.925, 0.957 | <0.001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.366 | 1.183, 1.578 | <0.001 |
| Diuretic dose >120 vs. ≤40 mg | 1.633 | 1.374, 1.939 | <0.001 |
| AF vs. No AF | 1.069 | 0.921, 1.242 | 0.3811 |
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.836 | 0.74, 0.945 | 0.0042 |
| Increasing age | 1.036 | 1.029, 1.043 | <0.001 |
| Female vs. male | 0.868 | 0.738, 1.02 | 0.0855 |
| Increasing systolic BP | 0.992 | 0.988, 0.995 | <0.001 |
| Increasing LVEF | 0.98 | 0.971, 0.988 | <0.001 |
| IHD vs. rest | 1.326 | 1.154, 1.522 | 0.0001 |
| NYHA III vs. NYHA II | 1.439 | 1.259, 1.645 | <0.001 |
| NYHA IV vs. NYHA II | 1.827 | 1.392, 2.398 | <0.001 |
| Previous angina | 0.939 | 0.809, 1.09 | 0.4078 |
| Increasing sodium | 0.941 | 0.925, 0.957 | <0.001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.366 | 1.183, 1.578 | <0.001 |
| Diuretic dose >120 vs. ≤40 mg | 1.633 | 1.374, 1.939 | <0.001 |
| AF vs. No AF | 1.069 | 0.921, 1.242 | 0.3811 |
IHD, ischaemic heart disease.
Multivariable analysis of risk of all-cause mortality in patients with AF vs. No AF at baseline
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.836 | 0.74, 0.945 | 0.0042 |
| Increasing age | 1.036 | 1.029, 1.043 | <0.001 |
| Female vs. male | 0.868 | 0.738, 1.02 | 0.0855 |
| Increasing systolic BP | 0.992 | 0.988, 0.995 | <0.001 |
| Increasing LVEF | 0.98 | 0.971, 0.988 | <0.001 |
| IHD vs. rest | 1.326 | 1.154, 1.522 | 0.0001 |
| NYHA III vs. NYHA II | 1.439 | 1.259, 1.645 | <0.001 |
| NYHA IV vs. NYHA II | 1.827 | 1.392, 2.398 | <0.001 |
| Previous angina | 0.939 | 0.809, 1.09 | 0.4078 |
| Increasing sodium | 0.941 | 0.925, 0.957 | <0.001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.366 | 1.183, 1.578 | <0.001 |
| Diuretic dose >120 vs. ≤40 mg | 1.633 | 1.374, 1.939 | <0.001 |
| AF vs. No AF | 1.069 | 0.921, 1.242 | 0.3811 |
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.836 | 0.74, 0.945 | 0.0042 |
| Increasing age | 1.036 | 1.029, 1.043 | <0.001 |
| Female vs. male | 0.868 | 0.738, 1.02 | 0.0855 |
| Increasing systolic BP | 0.992 | 0.988, 0.995 | <0.001 |
| Increasing LVEF | 0.98 | 0.971, 0.988 | <0.001 |
| IHD vs. rest | 1.326 | 1.154, 1.522 | 0.0001 |
| NYHA III vs. NYHA II | 1.439 | 1.259, 1.645 | <0.001 |
| NYHA IV vs. NYHA II | 1.827 | 1.392, 2.398 | <0.001 |
| Previous angina | 0.939 | 0.809, 1.09 | 0.4078 |
| Increasing sodium | 0.941 | 0.925, 0.957 | <0.001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.366 | 1.183, 1.578 | <0.001 |
| Diuretic dose >120 vs. ≤40 mg | 1.633 | 1.374, 1.939 | <0.001 |
| AF vs. No AF | 1.069 | 0.921, 1.242 | 0.3811 |
IHD, ischaemic heart disease.
Time dependent analysis of risk for all-cause mortality after new onset AF and NYHA class included in the model
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.918 | 0.795, 1.06 | 0.2418 |
| New-onset AF | 1.902 | 1.537, 2.354 | <0.0001 |
| NYHA II vs. NYHA I | 1.596 | 1.172, 2.174 | 0.003 |
| NYHA III vs. NYHA I | 3.414 | 2.501, 4.661 | <0.0001 |
| NYHA IV vs. NYHA I | 8.621 | 5.919, 12.558 | <0.0001 |
| Baseline covariables | |||
| Increasing age | 1.027 | 1.019, 1.035 | <0.0001 |
| Female vs. male | 0.841 | 0.702, 1.008 | 0.0615 |
| Increasing systolic BP | 0.993 | 0.989, 0.997 | 0.0008 |
| IHD vs. rest | 1.31 | 1.108, 1.548 | 0.0016 |
| Increasing LVEF | 0.979 | 0.969, 0.989 | 0.0001 |
| Previous angina | 1.01 | 0.85, 1.199 | 0.9136 |
| Increasing sodium | 0.947 | 0.929, 0.966 | <0.0001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.0001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.383 | 1.17, 1.635 | 0.0001 |
| Diuretic dose >120 vs. ≤40 mg | 1.541 | 1.258, 1.888 | <0.001 |
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.918 | 0.795, 1.06 | 0.2418 |
| New-onset AF | 1.902 | 1.537, 2.354 | <0.0001 |
| NYHA II vs. NYHA I | 1.596 | 1.172, 2.174 | 0.003 |
| NYHA III vs. NYHA I | 3.414 | 2.501, 4.661 | <0.0001 |
| NYHA IV vs. NYHA I | 8.621 | 5.919, 12.558 | <0.0001 |
| Baseline covariables | |||
| Increasing age | 1.027 | 1.019, 1.035 | <0.0001 |
| Female vs. male | 0.841 | 0.702, 1.008 | 0.0615 |
| Increasing systolic BP | 0.993 | 0.989, 0.997 | 0.0008 |
| IHD vs. rest | 1.31 | 1.108, 1.548 | 0.0016 |
| Increasing LVEF | 0.979 | 0.969, 0.989 | 0.0001 |
| Previous angina | 1.01 | 0.85, 1.199 | 0.9136 |
| Increasing sodium | 0.947 | 0.929, 0.966 | <0.0001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.0001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.383 | 1.17, 1.635 | 0.0001 |
| Diuretic dose >120 vs. ≤40 mg | 1.541 | 1.258, 1.888 | <0.001 |
Time dependent analysis of risk for all-cause mortality after new onset AF and NYHA class included in the model
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.918 | 0.795, 1.06 | 0.2418 |
| New-onset AF | 1.902 | 1.537, 2.354 | <0.0001 |
| NYHA II vs. NYHA I | 1.596 | 1.172, 2.174 | 0.003 |
| NYHA III vs. NYHA I | 3.414 | 2.501, 4.661 | <0.0001 |
| NYHA IV vs. NYHA I | 8.621 | 5.919, 12.558 | <0.0001 |
| Baseline covariables | |||
| Increasing age | 1.027 | 1.019, 1.035 | <0.0001 |
| Female vs. male | 0.841 | 0.702, 1.008 | 0.0615 |
| Increasing systolic BP | 0.993 | 0.989, 0.997 | 0.0008 |
| IHD vs. rest | 1.31 | 1.108, 1.548 | 0.0016 |
| Increasing LVEF | 0.979 | 0.969, 0.989 | 0.0001 |
| Previous angina | 1.01 | 0.85, 1.199 | 0.9136 |
| Increasing sodium | 0.947 | 0.929, 0.966 | <0.0001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.0001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.383 | 1.17, 1.635 | 0.0001 |
| Diuretic dose >120 vs. ≤40 mg | 1.541 | 1.258, 1.888 | <0.001 |
| . | RR . | 95% CI . | P-value . |
|---|---|---|---|
| Carvedilol vs. metoprolol | 0.918 | 0.795, 1.06 | 0.2418 |
| New-onset AF | 1.902 | 1.537, 2.354 | <0.0001 |
| NYHA II vs. NYHA I | 1.596 | 1.172, 2.174 | 0.003 |
| NYHA III vs. NYHA I | 3.414 | 2.501, 4.661 | <0.0001 |
| NYHA IV vs. NYHA I | 8.621 | 5.919, 12.558 | <0.0001 |
| Baseline covariables | |||
| Increasing age | 1.027 | 1.019, 1.035 | <0.0001 |
| Female vs. male | 0.841 | 0.702, 1.008 | 0.0615 |
| Increasing systolic BP | 0.993 | 0.989, 0.997 | 0.0008 |
| IHD vs. rest | 1.31 | 1.108, 1.548 | 0.0016 |
| Increasing LVEF | 0.979 | 0.969, 0.989 | 0.0001 |
| Previous angina | 1.01 | 0.85, 1.199 | 0.9136 |
| Increasing sodium | 0.947 | 0.929, 0.966 | <0.0001 |
| Increasing creatinine | 1.002 | 1.001, 1.003 | <0.0001 |
| Diuretic dose 41–120 vs. ≤40 mg | 1.383 | 1.17, 1.635 | 0.0001 |
| Diuretic dose >120 vs. ≤40 mg | 1.541 | 1.258, 1.888 | <0.001 |
References
Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group.
Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group.
Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA).
Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure.
Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction.
Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF).
Torp-Pedersen C, Møller M, Bloch-Thomsen PE, Køber L, Sandøe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm AJ. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group.
Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial—the Losartan Heart Failure Survival Study ELITE II.
Joglar JA, Acusta AP, Shusterman NH, Ramaswamy K, Kowal RC, Barbera SJ, Hamdan MH, Page RL. Effect of carvedilol on survival and hemodynamics in patients with atrial fibrillation and left ventricular dysfunction: retrospective analysis of the US Carvedilol Heart Failure Trials Program.
Lechat P, Hulot JS, Escolano S, Mallet A, Leizorovicz A, Werhlen-Grandjean M, Pochmalicki G, Dargie H. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial.
Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme.
Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Maderia HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis.
Deedwania PC, Singh BN, Ellenbogen K, Fisher S, Fletcher R, Singh SN. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators.
Crijns HJ, Tjeerdsma G, de Kam PJ, Boomsma F, van Gelder IC, van den Berg MP, van Veldhuisen DJ. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure.
Mathew J, Hunsberger S, Fleg J, McSherry F, Williford W, Yusuf S. Incidence, predictive factors, and prognostic significance of supraventricular tachyarrhythmias in congestive heart failure.
Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy.
Middlekauff HR, Stevenson WG, Stevenson LW, Saxon LA. Syncope in advanced heart failure: high risk of sudden death regardless of origin of syncope.
Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with atrial fibrillation and advanced heart failure.
Mahoney P, Kimmel S, DeNofrio D, Wahl P, Loh E. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure.
Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial.
Poole-Wilson PA, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Metra M, J Remme W, Swedberg K, Torp-Pedersen C. Rationale and design of the carvedilol or metoprolol European trial in patients with chronic heart failure: COMET.
Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients.
Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA. Improving survival for patients with advanced heart failure: a study of 737 consecutive patients.
Boutitie F, Boissel JP, Connolly SJ, Camm AJ, Cairns JA, Julian DG, Gent M, Janse MJ, Dorian P, Frangin G. Amiodarone interaction with beta-blockers: analysis of the merged EMIAT (European Myocardial Infarct Amiodarone Trial) and CAMIAT (Canadian Amiodarone Myocardial Infarction Trial) databases. The EMIAT and CAMIAT Investigators.
The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group.
Rationale and design of a study assessing treatment strategies of atrial fibrillation in patients with heart failure: the Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial.