-
PDF
- Split View
-
Views
-
Cite
Cite
Ruslan Malyuta, Marie-Louise Newell, Mikael Ostergren, Claire Thorne, Nadezhda Zhilka, Prevention of mother-to-child transmission of HIV infection: Ukraine experience to date, European Journal of Public Health, Volume 16, Issue 2, April 2006, Pages 123–127, https://doi.org/10.1093/eurpub/cki150
Close - Share Icon Share
Abstract
Background: Despite the availability of effective interventions for the prevention of mother-to-child transmission (PMTCT), questions remain regarding implementation of programmes in settings with limited resources. This article sets out to describe the first 2 years of the implementation of the national PMTCT programme in Ukraine. Methods: National data sources and data from a cohort of pregnant HIV-infected women delivering in 13 centres in Ukraine since 2000 were analysed. Results: Interventions for prevention of MTCT have been implemented as a national programme within Ukraine's well developed infrastructure for maternal and child health. Implementation of an ‘opt-out’ model of counselling and HIV testing in antenatal clinics resulted in a 97% uptake of women who agreed to be HIV tested. In 2002, ∼91% of HIV-positive pregnant women received ARV prophylaxis (mainly single-dose nevirapine or short-course zidovudine) for PMTCT. The MTCT rate has decreased from 30% in 2000 to 10% in 2002. The need to scale-up prevention interventions in pregnant women with risky behaviour and late access to medical services was identified in a review of the national programme in 2003. Conclusions: Further implementation of a comprehensive approach for the prevention of HIV infection in infants, including more extensive ART regimen, as recommended by WHO, would help Ukraine to achieve the strategic goal of virtual elimination of HIV infection in infants by 2010.
Vertical transmission is the main source of HIV infection in children with an estimated 2000 vertically-acquired HIV infections occurring daily globally, mostly in sub-Saharan Africa. Eastern Europe and Central Asia currently have the fastest growing epidemic in the world.1 Effective interventions for prevention of mother-to-child transmission (PMTCT) of HIV infection exist and where freely available, MTCT rates of 1–2% are achievable.2–4 The challenge is to provide available, accessible and affordable interventions to overcome the rapid increase in new HIV cases among children in countries with limited resources. The WHO Regional Office for Europe with other UN co-sponsors developed a Regional Strategic Framework for Prevention of HIV Infection in Infants.5 The goal set for the European region is virtual elimination (less than one HIV infected infant per 100 000 live births, and <2% of infants born to HIV-infected women acquiring HIV infection) of new HIV paediatric cases by 2010.
Ukraine was the first Eastern European country facing a dramatic spread of HIV/AIDS, which, in contrast to the HIV epidemic in African countries, has been driven by illicit injection drug usage (IDU).6,7 Incidence of HIV infection among IDUs has remained stable during the last 5 years, with 4000 cases officially registered annually, but has declined from 79%, in 1995–1998 to 58% in 1999–2002 among newly registered cases, with concomitant increases in heterosexually acquired cases. Risky sexual behaviour and low awareness of HIV prevention methods have contributed to the further spread beyond the IDUs to the general heterosexual population. By December 2003 more than 61 000 HIV-infected people had been registered in Ukraine, with more than 290 000 people living with HIV/AIDS, including 70 000 women. Over 7000 deliveries to HIV-infected women have been registered since 1997, and 2500 in 2003 alone. The prevalence of HIV among pregnant women in Ukraine is currently an estimated 0.5%, but is >1% in some regions (Ukrainian AIDS Center, 2003 unpublished data).
The National Programme to Fight HIV/AIDS was adopted in 1992. Prevention of HIV infection in infants became an integral part of this programme in 2001 and the implementation of the PMTCT programme was reviewed in mid-2003 by the Ministry of Health with experts from WHO, UNICEF and other international and national organizations. This article sets out to describe the first 2 years of the implementation of the PMTCT programme in Ukraine using national data sources and data from a cohort of pregnant HIV-infected women delivering in 13 centres in Ukraine since 2000.
Methods
Reproductive and maternal and child health-care services in Ukraine are implemented at a district level within a network of 466 antenatal clinics and 91 maternity houses. Out of 400 000 annual deliveries, the vast majority (99%) occur at maternity houses with supervision from trained health-care professionals (obstetrician, midwife, neonatologist). About two-thirds of women access antenatal care in the first trimester, but 10% of women receive no antenatal care.8 Despite a halving since 1995, abortion remains a major fertility control method, with an incidence of 828 per 1000 live births in 2002.
Description of the programme
The PMTCT programme has been integrated into existing maternal and child health care services, supervised by the Department of Health Care for Mothers and Children, with close collaboration from HIV/AIDS-specific services. The programme also includes development of legislative norms and regulations and training modules for health-care workers and policy makers. In 2001, the first national training module on PMTCT for health-care workers was initiated.
Antenatal testing and rapid testing at delivery
Access to voluntary counselling and testing is available in antenatal clinics for all pregnant women. HIV testing is free of charge and included in the routine package of antenatal screening tests, including syphilis and viral hepatitis, with an ‘opt-out’ strategy. HIV enzyme-linked immunosorbent assay (ELISA) screening tests are performed in 69 laboratories, with confirmation testing in seven referral laboratories. HIV screening is performed twice during pregnancy; positive results are confirmed by two ELISA tests; western blot is used if ELISA results are inconclusive. Antenatal testing is provided with informed consent, and the right of women to refuse the HIV test is discussed during counselling. Pretest counselling is conducted by midwives and obstetricians, who ideally have undertaken a special counselling training course. Women with positive tests are referred to a specialist within their local AIDS centre, where they receive post-test counselling, including information on the laws pertaining to HIV-positive individuals in Ukraine, and preventing transmission of HIV to partners and infants. Women are encouraged to invite their partners for HIV counselling and testing. Since 2001, in three pilot regions, women without antenatal care presenting in labour are offered rapid HIV testing (using Multispot HIV-1/HIV-2 rapid test kits); this strategy was implemented nationally in 2003.
Management of HIV-infected women: before, during and after delivery
HIV-infected women have about eight routine antenatal visits, the same as uninfected women. An HIV-infected woman can deliver at any maternity service; alternatively, she can choose to be followed at the PMTCT reference centre, where staff are more experienced. Women are cared for by reproductive health specialists or local gynaecologists together with an HIV specialist from the AIDS centre.
Ukraine implemented ARV prophylaxis of MTCT on a large scale in 2001. Protocols included maternal course of zidovudine from 36 weeks gestation until delivery9 and/or single-dose nevirapine for mother and infant.10 ARV drugs were provided by donations from pharmaceutical companies and international charity organizations.
As a result of concerns regarding the potential increased risk of infectious complications after delivery,11,12 elective Caesarean section (CS) was not adopted as a standard of care for HIV-infected pregnant women in the national PMTCT protocol. In 2002 the CS rate was 12.4% among infected women, comparable to the 12.1% in the general population. Follow-up of infants born to HIV-infected mothers is provided by local paediatricians with the HIV specialist from the AIDS centre. The programme supplies free milk formula for children born to HIV-infected mothers, with funding from local budgets. Owing to the limited resources, early RNA or DNA PCR diagnosis of infant HIV status is not currently widely available. Diagnosis of HIV is based on an ELISA test at 18 months of age.
Monitoring of the programme
The implementation of the PMTCT programme is monitored by the Department of Mother and Child Health of the Ministry of Health, with the Republican Centre for the Prevention and Fight Against HIV/AIDS. The following indicators are routinely collected in the health institutions: proportion of all women receiving VCT during pregnancy, proportion of pregnant women identified as HIV infected, number of deliveries among infected women, mode of delivery, proportion of infected pregnant women receiving ARV prophylaxis for MTCT, HIV status of infants born to infected mothers and method of infant feeding.
In mid-2003 the Ministry of Health reviewed the first 2 years of implementation of the PMTCT programme with expert participation from WHO, UNICEF, and other international and national organizations. Assessment included field visits in urban and rural areas in four regions with high, mid and low prevalence, and desk review of available Federal documents and studies; evaluation included organizational framework, policy and protocols, human resources and quality of service, management systems and supplies and community awareness, mobilization, and support. The review assessed achievements to date and also identified constraints and feasible solutions to these constraints, and these, together with recommendations for future directions, will be incorporated in the national multisectoral program for 2005–2011.
Cohort of HIV-infected pregnant women
Data on 860 women identified as HIV-infected through antenatal testing or rapid testing during labour delivering between January 2000 and January 2004 were prospectively collected in 13 maternity hospitals in Southern Ukraine. The selection of Odessa (n = 7), Simferopol (n = 1) and Mycolaiv (n = 5) was based on their high HIV prevalence. All HIV-infected women were invited to participate in the study, with verbal consent. Information relating to socio-demographic and clinical characteristics and mode of acquisition was collected by the woman's clinician. Data management and analysis were carried out in Microsoft Access.
Results
Antenatal HIV testing
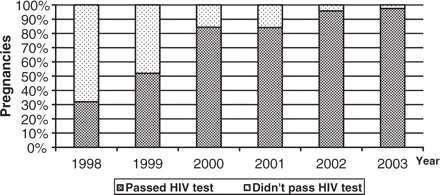
Implementation of the antenatal HIV testing strategy resulted in a significant increase in the proportion of pregnant women agreeing to be tested from 32% in 1998 to 97% in 2003 (χtrend2 > 1000; P < 0.001) (Figure 1). In 2002, 12% (253/2022) of infected pregnant women were identified during labour or soon after delivery. In the cohort study, information on the timing of diagnosis was available for 777 (90%) women, of whom 142 (18%) were identified as HIV-infected before the pregnancy, 521 (67%) as a result of antenatal testing and 114 (15%) through rapid testing during labour.
Trends in HIV testing during pregnancy and delivery, 1998–2003.
In 2002, a total of 392 020 (96%) pregnant women were tested for HIV antenatally or during labour, of whom 2022 (0.5%) were found to be infected, a prevalence of 5.16 per 1000 pregnant women. Of the 2022 infected pregnant women, 1334 (66%) had a live birth and 211 (10%) continued their pregnancy and delivered the next year. Four hundred and seventy-seven (24%) terminated their pregnancies (358 per 1000 live births).
Characteristics of HIV-infected pregnant women
Within the cohort study, most women were young, married or cohabiting, and nulliparous (Table 1). Although over one-third of women were IDUs themselves or had sexual partners with a history of IDU, the largest group were women who most likely had acquired the infection heterosexually, and did not specify belonging to any high-risk group (Table 1).
Maternal information on the 860 mother–child pairs in the cohort study
. | n (%) . | |
|---|---|---|
| Maternal age, years (n = 837) | ||
| Median | 25.1 | |
| Range | 14–41 | |
| Ethnicity (n = 843) | ||
| White | 828 (98) | |
| Oriental | 5 | |
| Asian | 5 | |
| Black | 1 | |
| Other | 4 | |
| Marital status | ||
| Single | 128 (15) | |
| Married | 374 (43) | |
| Cohabiting | 318 (37) | |
| Divorced/widowed | 12 (1) | |
| Missing | 28 (3) | |
| Parity (n = 828) | ||
| 0 | 484 (58) | |
| 1 | 255 (31) | |
| 2 | 61 (7) | |
| ≥3 | 28 (3) | |
| Previous termination of pregnancy (n = 829) | ||
| 0 | 477 (58) | |
| 1 | 172 (21) | |
| 2 | 94 (11) | |
| ≥3 | 86 (10) | |
| Mode of acquisition of HIV infection | ||
| IDU | 74 (9) | |
| IDU and IDU sexual partner | 168 (20) | |
| IDU sexual partner | 163 (19) | |
| Other high risk sexual partner | 55 (6) | |
| Blood transfusion | 4 | |
| Occupational exposure | 3 | |
| Unspecified risk group | 393 (46) | |
| Timing of last injecting drug use (n = 242) | ||
| Ex-user | 103 (42) | |
| IDU in pregnancy | 86 (36) | |
| Last IDU timing unspecified | 53 (22) | |
. | n (%) . | |
|---|---|---|
| Maternal age, years (n = 837) | ||
| Median | 25.1 | |
| Range | 14–41 | |
| Ethnicity (n = 843) | ||
| White | 828 (98) | |
| Oriental | 5 | |
| Asian | 5 | |
| Black | 1 | |
| Other | 4 | |
| Marital status | ||
| Single | 128 (15) | |
| Married | 374 (43) | |
| Cohabiting | 318 (37) | |
| Divorced/widowed | 12 (1) | |
| Missing | 28 (3) | |
| Parity (n = 828) | ||
| 0 | 484 (58) | |
| 1 | 255 (31) | |
| 2 | 61 (7) | |
| ≥3 | 28 (3) | |
| Previous termination of pregnancy (n = 829) | ||
| 0 | 477 (58) | |
| 1 | 172 (21) | |
| 2 | 94 (11) | |
| ≥3 | 86 (10) | |
| Mode of acquisition of HIV infection | ||
| IDU | 74 (9) | |
| IDU and IDU sexual partner | 168 (20) | |
| IDU sexual partner | 163 (19) | |
| Other high risk sexual partner | 55 (6) | |
| Blood transfusion | 4 | |
| Occupational exposure | 3 | |
| Unspecified risk group | 393 (46) | |
| Timing of last injecting drug use (n = 242) | ||
| Ex-user | 103 (42) | |
| IDU in pregnancy | 86 (36) | |
| Last IDU timing unspecified | 53 (22) | |
Maternal information on the 860 mother–child pairs in the cohort study
. | n (%) . | |
|---|---|---|
| Maternal age, years (n = 837) | ||
| Median | 25.1 | |
| Range | 14–41 | |
| Ethnicity (n = 843) | ||
| White | 828 (98) | |
| Oriental | 5 | |
| Asian | 5 | |
| Black | 1 | |
| Other | 4 | |
| Marital status | ||
| Single | 128 (15) | |
| Married | 374 (43) | |
| Cohabiting | 318 (37) | |
| Divorced/widowed | 12 (1) | |
| Missing | 28 (3) | |
| Parity (n = 828) | ||
| 0 | 484 (58) | |
| 1 | 255 (31) | |
| 2 | 61 (7) | |
| ≥3 | 28 (3) | |
| Previous termination of pregnancy (n = 829) | ||
| 0 | 477 (58) | |
| 1 | 172 (21) | |
| 2 | 94 (11) | |
| ≥3 | 86 (10) | |
| Mode of acquisition of HIV infection | ||
| IDU | 74 (9) | |
| IDU and IDU sexual partner | 168 (20) | |
| IDU sexual partner | 163 (19) | |
| Other high risk sexual partner | 55 (6) | |
| Blood transfusion | 4 | |
| Occupational exposure | 3 | |
| Unspecified risk group | 393 (46) | |
| Timing of last injecting drug use (n = 242) | ||
| Ex-user | 103 (42) | |
| IDU in pregnancy | 86 (36) | |
| Last IDU timing unspecified | 53 (22) | |
. | n (%) . | |
|---|---|---|
| Maternal age, years (n = 837) | ||
| Median | 25.1 | |
| Range | 14–41 | |
| Ethnicity (n = 843) | ||
| White | 828 (98) | |
| Oriental | 5 | |
| Asian | 5 | |
| Black | 1 | |
| Other | 4 | |
| Marital status | ||
| Single | 128 (15) | |
| Married | 374 (43) | |
| Cohabiting | 318 (37) | |
| Divorced/widowed | 12 (1) | |
| Missing | 28 (3) | |
| Parity (n = 828) | ||
| 0 | 484 (58) | |
| 1 | 255 (31) | |
| 2 | 61 (7) | |
| ≥3 | 28 (3) | |
| Previous termination of pregnancy (n = 829) | ||
| 0 | 477 (58) | |
| 1 | 172 (21) | |
| 2 | 94 (11) | |
| ≥3 | 86 (10) | |
| Mode of acquisition of HIV infection | ||
| IDU | 74 (9) | |
| IDU and IDU sexual partner | 168 (20) | |
| IDU sexual partner | 163 (19) | |
| Other high risk sexual partner | 55 (6) | |
| Blood transfusion | 4 | |
| Occupational exposure | 3 | |
| Unspecified risk group | 393 (46) | |
| Timing of last injecting drug use (n = 242) | ||
| Ex-user | 103 (42) | |
| IDU in pregnancy | 86 (36) | |
| Last IDU timing unspecified | 53 (22) | |
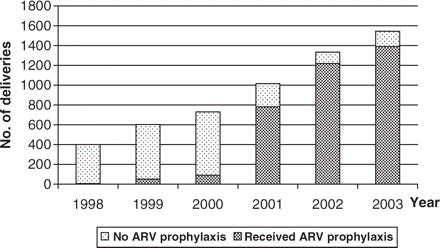
Prophylactic antiretrovirals
Figure 2 shows the dramatic increase in receipt of ARV prophylaxis among HIV-infected pregnant women and their infants over time, from <10% in 1999 to 91% in 2002 (χtrend2 = 1164; P < 0.001). Of the 782 women receiving prophylaxis in 2001, 336 (43%) received short-course zidovudine prophylaxis and 446 (57%) single-dose nevirapine, while in 2002 among the 1219 women receiving prophylaxis, the proportion receiving zidovudine increased to 713 (59%), with the remaining 506 (42%) receiving single-dose nevirapine or a combination of two courses. In the cohort study, single-dose nevirapine for the mother and neonate was the most commonly used prophylaxis (Table 2).
Cohort study: obstetric and perinatal information
. | n (%) . | |
|---|---|---|
| Mode of delivery (n = 859) | ||
| Vaginal | 546a (64) | |
| Emergency CS | 23 (3) | |
| Elective CS | 280 (33) | |
| Gestational age, weeks | ||
| Median (range) | 39 (27–42) | |
| <37 weeks | 85 (10) | |
| Birthweight, g (n = 854) | ||
| Median (range) | 3080 (1200–4400) | |
| ≥2500 g | 740 (87) | |
| <2500 g | 114 (13) | |
| Proportion (%) of women who were ‘missed’ by antenatal care and were first tested HIV-positive at the time of delivery in 13 clinical sites | ||
| Median (range) | 18 (5–43) | |
| Prophylactic ARV (n = 702) | ||
| Maternal SC ZDV + neonatal ZDV | 24 (3) | |
| Maternal SC ZDV + SD NVP | 133 (19) | |
| SD NVP only (mother and infant) | 498 (71) | |
| SD NVP + neonatal ZDV | 47 (7) | |
. | n (%) . | |
|---|---|---|
| Mode of delivery (n = 859) | ||
| Vaginal | 546a (64) | |
| Emergency CS | 23 (3) | |
| Elective CS | 280 (33) | |
| Gestational age, weeks | ||
| Median (range) | 39 (27–42) | |
| <37 weeks | 85 (10) | |
| Birthweight, g (n = 854) | ||
| Median (range) | 3080 (1200–4400) | |
| ≥2500 g | 740 (87) | |
| <2500 g | 114 (13) | |
| Proportion (%) of women who were ‘missed’ by antenatal care and were first tested HIV-positive at the time of delivery in 13 clinical sites | ||
| Median (range) | 18 (5–43) | |
| Prophylactic ARV (n = 702) | ||
| Maternal SC ZDV + neonatal ZDV | 24 (3) | |
| Maternal SC ZDV + SD NVP | 133 (19) | |
| SD NVP only (mother and infant) | 498 (71) | |
| SD NVP + neonatal ZDV | 47 (7) | |
a: Three forceps deliveries
SC = short course (median from 34 weeks gestation); SD = single dose; ZDV = zidovudine; NVP = nevirapine
Cohort study: obstetric and perinatal information
. | n (%) . | |
|---|---|---|
| Mode of delivery (n = 859) | ||
| Vaginal | 546a (64) | |
| Emergency CS | 23 (3) | |
| Elective CS | 280 (33) | |
| Gestational age, weeks | ||
| Median (range) | 39 (27–42) | |
| <37 weeks | 85 (10) | |
| Birthweight, g (n = 854) | ||
| Median (range) | 3080 (1200–4400) | |
| ≥2500 g | 740 (87) | |
| <2500 g | 114 (13) | |
| Proportion (%) of women who were ‘missed’ by antenatal care and were first tested HIV-positive at the time of delivery in 13 clinical sites | ||
| Median (range) | 18 (5–43) | |
| Prophylactic ARV (n = 702) | ||
| Maternal SC ZDV + neonatal ZDV | 24 (3) | |
| Maternal SC ZDV + SD NVP | 133 (19) | |
| SD NVP only (mother and infant) | 498 (71) | |
| SD NVP + neonatal ZDV | 47 (7) | |
. | n (%) . | |
|---|---|---|
| Mode of delivery (n = 859) | ||
| Vaginal | 546a (64) | |
| Emergency CS | 23 (3) | |
| Elective CS | 280 (33) | |
| Gestational age, weeks | ||
| Median (range) | 39 (27–42) | |
| <37 weeks | 85 (10) | |
| Birthweight, g (n = 854) | ||
| Median (range) | 3080 (1200–4400) | |
| ≥2500 g | 740 (87) | |
| <2500 g | 114 (13) | |
| Proportion (%) of women who were ‘missed’ by antenatal care and were first tested HIV-positive at the time of delivery in 13 clinical sites | ||
| Median (range) | 18 (5–43) | |
| Prophylactic ARV (n = 702) | ||
| Maternal SC ZDV + neonatal ZDV | 24 (3) | |
| Maternal SC ZDV + SD NVP | 133 (19) | |
| SD NVP only (mother and infant) | 498 (71) | |
| SD NVP + neonatal ZDV | 47 (7) | |
a: Three forceps deliveries
SC = short course (median from 34 weeks gestation); SD = single dose; ZDV = zidovudine; NVP = nevirapine
Mode of delivery
In 2002, the elective CS rate in the centres in the cohort study was 32% (87/274), significantly higher than the remaining deliveries nationally (78/1060; 7%) (χ2 = 119.4; P < 0.001). These differences reflect the fact that, although national guidelines did not recommend use of elective CS to prevent MTCT, several pilot medical centres and university clinics had already started the broader use of elective CS for HIV-infected women in order to test the feasibility and safety of this intervention in Ukraine.
The neonatal and post-natal periods
Data from the cohort study show good perinatal outcome among the mother–child pairs enrolled with regard to prematurity and birth weight (Table 2). Non-breast-feeding of the child is not stigmatizing in Ukraine and 99% of HIV-positive women choose to formula feed. In the cohort study, only four women (0.5%) opted to breast feed their infants; all were aware of their infection status.
Discussion
The MTCT rate in Ukraine decreased from 27.5% in 2000 to 10% in 2002,13 reflecting the success of the national PMTCT programme, which is partly attributable to its coordinated effort in preventive, clinical and social activities, involving a variety of stakeholders. Furthermore, the focus on supporting NGOs working in HIV prevention, care and support of people living with HIV/AIDS, and reducing the epidemic's negative impact has been very important. Cooperation between the Ministry of Health and UN agencies is an important asset to the programme and the UN has facilitated consolidation of the efforts of NGOs and international donor agencies. In 2003 Ukraine received a US$92 million grant from Global Fund to Fight AIDS, TB and Malaria (GFATM). Simultaneously, ARV generic drugs became registered in Ukraine, allowing the opportunity for scaling up ARV treatment programmes.
Improving access to prophylactic MTCT interventions was facilitated by the integration of the programme into the pre-existing health-care system and the optimal selection of health care institutions providing services to HIV-infected women and their families. The selected antenatal testing and counselling ‘opt-out’ strategy has proved to be effective elsewhere.14 Although this approach is beneficial, with HIV testing becoming a routine antenatal procedure, avoiding stigmatizing any particular group, and a high testing uptake, it has limitations. In a recent study among HIV-infected pregnant women and mothers, more than half were tested antenatally without informed consent and/or pre-test counselling:15 three-quarters received post-test counselling, but in about half of cases this provided incomplete information, often in a way that women found difficult to understand. The plan is thus now to improve and expand the provision of quality counselling and antenatal testing, with the aim of offering voluntary HIV testing and counselling to all pregnant women, whilst providing continuous monitoring and evaluation. Non-return for test results has been a limiting factor in the optimal implementation of some PMTCT programmes in Africa,16,17 but here, almost 100% of women who were tested for HIV returned for their test results (data not shown), reflecting the organization of HIV testing within the antenatal care.
Many HIV-infected women in Ukraine are also socially disadvantaged18 and/or have co-morbidities. A significant proportion do not receive antenatal care, often presenting in labour with unknown HIV status. This represents a challenge for HIV diagnosis and administration of PMTCT interventions, and potentially higher risk of transmission to infant. Data from the cohort study indicate that overall around one-fifth of women were first tested as HIV-positive at the time of delivery. Around one-third of the women in the cohort study had a IDU history, and at least one-third of these were actively using drugs during pregnancy. The actual numbers could be higher, but due to the stigma associated with IDU it may have been under-reported as a mode of acquisition, as we relied on self-report, clinical observation and drug withdrawal symptoms in the infant for information on IDU. Pregnant women who are injecting street drugs are a hard to reach group with regard to interventions for PMTCT.18 Chaotic lifestyle, poor health-seeking behaviour and stigma related to drug addiction frequently results in late presentation to the health-care system, often in advanced labour. Rapid testing provided an opportunity for administration of nevirapine to the mother in 50% of cases and post-exposure prophylaxis for 75% of infants. There is a demand to develop special services for IDU pregnant women to improve their adherence to ARV medications.19 Pilot projects with substitution treatment in Ukraine are planned for 2005, including one among IDU pregnant women. A high rate of infant abandonment has been documented among HIV-positive IDU women,20 and in the cohort study here, 28 (30%) of the 97 children born to active IDUs were abandoned by their parents soon after delivery, reflecting poor access to family planning services and use of contraception by this group.
In 2004, the Ukraine Ministry of Health adopted new guidelines on use of ARV during pregnancy, based on WHO recommendations.5 Implementation of these guidelines will allow pregnant women to receive ARV drugs for her own health needs as well as for PMTCT. Although overall the elective CS rate among HIV-infected women in Ukraine was relatively low, in certain pilot centres up to one-third of infected women are now being delivered by elective CS. National guidelines are currently being updated regarding mode of delivery among pregnant HIV-infected women, and elective CS is to be recommended as the first choice method with the informed consent of the woman.21–25 The multidisciplinary approach taken in the management of pregnant HIV-infected women enabled their access to services including the diagnosis and treatment of reproductive tract infections, contraception and follow-up of their HIV disease.
Despite relatively high prevalence of HIV infection among pregnant women, Ukraine is still faced with a lack of capacity for early diagnosis of HIV infection in infants using virological methods. This creates an obstacle for appropriate care of the HIV-infected child, including decisions related to initiating antiretroviral therapy and/or other appropriate treatment for infected children. With the support from GFATM, the Ministry of Health is planning to implement PCR diagnosis in referral laboratories.
As cohort data presented here suggest, a large proportion of infected women in Ukraine have acquired HIV heterosexually but do not report having partners with high-risk behaviours. This reflects the shift in the epidemic towards the general heterosexual population, and is consistent with the high incidence of sexually transmitted infections in Ukraine.6,26
UNAIDS estimates that unless urgent measures to fight the epidemic are implemented, 1.5 million people will be living with HIV/AIDS in Ukraine, two-thirds of them of reproductive age, by 2010. The challenges of the HIV epidemic as it applies to women and children are varied. It is necessary to mobilize the support of policy makers, health-care workers and the public, and to move from a vertical to a horizontal structure of care. Access to testing and counselling services must be universal, and the quality of services of a high standard. Large-scale implementation of psychosocial support for HIV-infected pregnant women with involvement of volunteers from NGOs is needed. Currently, members of the ALL-Ukrainian Network of People Living with HIV/AIDS are actively involved in development of national programmes for care and support of HIV-positive people. This organization helps to improve access to prophylaxis interventions among marginalized and vulnerable women and to prevent stigmatization and discrimination of persons affected by HIV.
The goals of the next PMTCT programme go beyond decreasing the MTCT risk to a minimum, and aim to achieve the strategic goal of virtual elimination of HIV infection in infants. In order to achieve this goal a comprehensive approach is needed and a four-pronged strategy has been suggested, including primary prevention of HIV infection in women, contraception and family planning in HIV-infected women, prevention of MTCT, and care and support for HIV-infected women and their family members.5
The health-care system in Ukraine is similar to many former Soviet countries. Implementation of HIV prevention in infants with the same model as in Ukraine could have a positive impact on prevention of the spread of HIV epidemic among infants in these countries.
The aim was to describe the implementation of the prevention of mother-to-child transmission of HIV (MTCT) programme in Ukraine in 2001–2002.
The programme provides universal access to antenatal testing and provision of short-course zidovudine prophylaxis and single-dose nevirapine.
There has been rapid uptake of interventions and MTCT rates declined substantially, from 30% in 2000 to 10% in 2002.
Improved access/uptake of interventions among women from vulnerable groups with late presentation to the health services is a challenge.
A comprehensive approach is needed to achieve the UN goal of virtual elimination of HIV infection in infants by 2010.
References
Dorenbaum A, Cunningham CK, Gelber RD, et al. International PACTG 316 Team. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial.
Ioannidis JP, Abrams EJ, Ammann A, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus load <1000 copies/ml.
Mandelbrot L, Landreu-Mascaro A, Rekacewicz C, et al. Lamivudine–zidovudine combination for prevention of maternal–infant transmission of HIV-1.
Strategic Framework for Prevention of HIV Infection in Infants in Europe. Copenhagen: World Health Organization Regional Office for Europe,
HIV/AIDS in Eastern Europe and the Commonwealth of Independent States—reversing the epidemic: facts and policy options. Bratislava: United Nations Development Programme,
Goldberg H, Melnikova N, Buslayeva E, Zakhozha V. 1999 Ukraine Reproductive Health Survey, final report. Kiev International Institute of Sociology, Centers for Disease Control and Prevention Division of Reproductive Health and the United States Agency for International Development,
Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial.
Bulterys M, Chao A, Dushimana A, Saah A. Fatal complications after Caesarian section in HIV-infected women.
Semprini AE, Castagni C, Ravizza M, et al. The incidence of complications after caesarean section in 156 HIV-positive women.
Sherbinskaya A, Kruglov Y, Marcinovskaya V, et al. HIV/AIDS among children in Ukraine. Conference abstract. XV International AIDS Conference, July 11–16,
Gayatri C, Jayaraman, Jutta K, et al. Mandatory reporting of HIV infection and opt-out prenatal screening for HIV infection: effect on testing rates.
Medrek M, Eckman AK, Yaremenko O, et al. Problems HIV-positive women face accessing reproductive health services in Ukraine. Conference abstract. XV International AIDS Conference, July 11–16,
Temmerman M, Quaghabeur A, Mwanyumba F, Mandaliya K. Mother-to-child HIV transmission in resource poor settings: how to improve coverage?
Stringer EM, Sinkala M, Stringer JS, et al. Prevention of mother-to- child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia.
Aryaev N, Malyuta R, Semenenko I. Drug addiction and mother-to-child HIV transmission prevention programmes in the South Ukraine. Conference abstract. XV International AIDS Conference, July 11–16,
National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction Effective Medical Treatment of Opiate Addiction.
Khaldeeva N, Hillis SD, Vinogradova E, et al. HIV-1 seroprevalence rates in women and relinquishment of infants to the state in St Petersberg, Russia, 2002.
International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1.
European Collaborative Study. Vertical transmission of HIV-1: maternal immune status and obstetric factors.
European Collaborative Study. Caesarean section and the risk of vertical transmission of HIV-1 infection.
Dunn DT, Newell ML, Mayaux MJ, et al. Mode of delivery and vertical transmission of HIV-1: a review of prospective studies.
Pregnancy and HIV infection: a European consensus on management: Executive summary.




Comments