-
PDF
- Split View
-
Views
-
Cite
Cite
David V. Espino, Oralia V. Bazaldua, Raymond F. Palmer, Charles P. Mouton, Michael L. Parchman, Toni P. Miles, Kyriakos Markides, Suboptimal Medication Use and Mortality in an Older Adult Community-Based Cohort: Results From the Hispanic EPESE Study, The Journals of Gerontology: Series A, Volume 61, Issue 2, February 2006, Pages 170–175, https://doi.org/10.1093/gerona/61.2.170
Close - Share Icon Share
Abstract
Background. Numerous methods have been used to evaluate medication management quality in older adults; however, their predictive validities are unknown. Major medication quality indicators include polypharmacy, drug–drug interactions, and inappropriate medication use. To date, no study has attempted to evaluate the three approaches systematically or the effect of each approach on mortality in a Hispanic population. Our objective was to evaluate the relationship between polypharmacy, drug–drug interactions, and inappropriate medication use on the mortality of a community-based population of Mexican American older adults.
Methods. We used a life table survival analysis of a longitudinal survey of a representative sample of 3050 older Mexican Americans of whom 1823 were taking prescription and over-the-counter medications.
Results. After adjustment for relevant covariates, use of more than four different medications (polypharmacy) was independently associated with mortality. The presence of major drug interactions and the use of inappropriate medications were not significantly associated with mortality in our study sample.
Conclusion. Polypharmacy (>4 medications) is significantly associated with mortality in Mexican American older adults. This community-based study is the first to demonstrate a direct association between polypharmacy and mortality in this population.
Medication management represents one of the most important health care issues for older adults in the United States. Recently, medication management was identified as a “priority area for national action” by the Institute of Medicine (1). Medication-related errors are common and are estimated to account for more than 7000 deaths annually (2,3). In 1994, adverse drug reactions were the sixth leading cause of death in hospitalized patients (4). In the outpatient setting, 25% of patients reported having an adverse drug event, of which 13% were considered serious (5). In the year 2000, it was estimated that costs due to medication-related problems exceeded $177 billion (6). Because adults aged 65 years and older consume more than 30% of prescription medications (7), suboptimal medication management is a major patient safety concern in older adults.
Although a number of methods have been used to evaluate medication management quality in older adults, their predictive validities are unknown. Also, there is no agreement on a standard definition for the use of medications that leads to unintended harm. Suboptimal medication management has been defined as overuse, underuse, erratic use, or contraindicated use of a prescribed or nonprescribed medication (8). Other terms commonly used are adverse drug events, drug-related problems, and adverse drug reactions. Medication outcomes most commonly studied in older adult populations are those related to polypharmacy, drug–drug interactions, and the use of inappropriate drugs. Therefore, we use the term suboptimal medication use as defined by any one of the following: (i) polypharmacy—use of more than four medications, (ii) drug–drug interactions—presence of any drug–drug interaction, or (iii) the presence of inappropriate medication use as defined by the Beer's criteria (9).
Potential causes of poor outcomes due to medications are numerous. Polypharmacy has been associated with a higher risk of adverse events, poor patient compliance, higher health care costs, and increased hospitalizations (1,10,11). Use of more than a single medication also carries the potential of drug–drug interactions. Drug–drug interactions are common among older ambulatory adults, and more than 100,000 different drug–drug interactions have been identified as potentially serious (12). Additionally, geriatric care experts have developed an “inappropriate” medication list consisting of medications considered unsuitable for older adult patients due to the high risk of unintended harm (9,13). The prevalence of inappropriate medication use in older adults has been estimated to be as high as 40% (11). Each of these indicators illustrates potential suboptimal medication management.
Studies have approached quality indicators for suboptimal medication management in various ways including inappropriate medication use and potential drug–drug interactions. Each approach has individually been shown to be a significant factor in determining adverse outcomes from medication use. To date, no study has attempted to evaluate the effect of each quality indicator on mortality in a community-based older adult population. This article describes the impact of polypharmacy, drug-drug interactions, and inappropriate medication use on the mortality of a selected population of community-based Mexican American older adults living in the southwestern United States.
Methods
Participants
The Hispanic Established Populations for Epidemiologic Studies of the Elderly (EPESE) is an ongoing longitudinal study of Mexican American older adults between the ages of 65 and 99. The study design and sampling have been described previously (14). In brief, the sample was drawn using area probability sampling procedures to represent the Mexican American older adult population residing in Texas, New Mexico, Colorado, Arizona, and California. In 1993, the study team completed in-home contacts with the participants that included medication information. There were 3050 participants interviewed and evaluated in their own homes by trained interviewers. Follow-up in-home evaluations were done approximately 2 years apart with the most recent contact being approximately 8 years after the original contact. Of the 3050 baseline interviewees, 1823 reported using medication and 1227 did not. Eight-year mortality data were used in the present study. There were 940 deaths (30.8%) since baseline. For deceased persons, a brief proxy interview was obtained that included information on the time and place of death, causes of death, and any hospitalizations or nursing home admissions. Validation of death was confirmed by a National Death Index search. Research protocol was approved by the UTHSCSA and UTMB institutional review boards, and all participants gave informed oral consent.
Medication Definitions
We define suboptimal medication use as any of the following:
Polypharmacy
Polypharmacy is defined as the use of more than four medications. This number was chosen for clarity of presentation and to be consistent with prior definitions of polypharmacy (15–18). The total number of prescription and over-the-counter medications taken by each participant were counted, and participants were categorized into groups taking 1, 2, 3, 4, or >4 medications. Combination products were counted as more than one medication by the total number of active ingredients.
Drug–drug interactions
Potential adverse drug–drug interactions were determined for each participant by use of the Micromedex Intranet Knowledge Base System (19). This system was selected because it is comprehensive, widely available, and interactive. The Micromedex system, part of the mobile Physicians Desk Reference, is designed to assist the clinician in interpreting interaction data. The Micromedex system used an expert panel to identify drug interactions and classify them into three groups. Each participant's medications were entered from the Hispanic EPESE database into the Micromedex system: Drug interactions were then categorized into major, moderate, or minor drug–drug interactions by using the following criteria developed by the Micromedex system: (i) Major: The adverse interaction may be any interaction that is contraindicated, life-threatening, and/or requires medical intervention to minimize or prevent serious adverse effects. Examples include the use of erythromycin with amiodarone or metoprolol with verapamil. (ii) Moderate: The interaction may result in an exacerbation of the patient's condition and/or require an alteration in therapy. (iii) Minor: The interaction would have limited clinical effects. Manifestations may include an increase in the frequency or severity of side effects but generally would not require a major alteration in therapy.
“Inappropriate” medication use
Each participant's medication list was evaluated for use of “inappropriate” medications by using the Beer's criteria for inappropriate medication use (9), and participants were classified into those using inappropriate medications and those not using inappropriate medications. Beer's criteria include a list of medications considered inappropriate by an expert panel because they are either ineffective or present unnecessary high risk. Examples include the use of carisoprodol, chlorpropamide, ticlopidine, and flurazepam.
Other Variables
Age-adjusted mortality
Mortality was evaluated at follow-up visits every 2 years for a total of 8 years, and was validated by using the National Death Index. Mortality rates for those participants who met criteria for suboptimal medication use, compared to those who did not, were age-adjusted using the direct method of adjustment. The 1990 U.S. Census data, as the Census time closest to the EPESE medication data collection, was used as the standard population for age distribution of Mexican Americans age 65 years or older.
Sociodemographic variables
Sociodemographic variables collected included age, gender, date of birth, current household income, current employment status, and acculturation. Acculturation was measured using Hazuda's algorithm (20).
Illness severity
Instrumental Activities of Daily Living (IADL) and self-reported health status were used to estimate illness severity. IADLs were assessed using the modified Older American Resource Scale (OARS) (21). For self-reported health status, participants were asked to rate their current health status as excellent, good, fair, or poor.
Disease states
We assessed the presence of chronic illnesses with a self-reported condition checklist used previously in the EPESE studies. The major disease states listed were those defined by the National Center for Health Statistics as the leading causes of death in the United States (22). In order, these are: cardiovascular disease, neoplasms, cerebrovascular disease, chronic obstructive pulmonary disease, and diabetes mellitus. Hypertension was also included due to its major impact on morbidity and medication use in the Mexican American population. We examined each disease separately, as individual diseases have differential impact on medication use and on mortality. Chronic obstructive pulmonary disease was not included in the EPESE baseline evaluation and was therefore excluded from the analyses.
Statistical Analysis
Life table survival estimates were obtained using SAS software (23). The survival function S(t) was calculated as S(t) = 1 − f(t), where f(t) indicates the death rate as a function of time. Homogeneity of the estimated survival curves between the various medication usage groups were tested using the Wilcoxon signed rank and log-rank test statistics (24). These statistics test the null hypothesis that the rates of decline in each group are not statistically different. Survival distribution plots were drawn for visual inspection. In addition, Cox proportional hazards regression models (25) were used to control for gender, age, educational level, illness severity, and six chronic comorbid disease states.
Results
Sample Characteristics
The demographic characteristics (Table 1) show that 40.2% (n = 1227) of participants used no medications. Those participants who used medications were more likely to be female, have poor self-reported health, have ADL limitations, and suffer differentially from the five chronic diseases examined.
Unadjusted Survival Models
The unadjusted proportional hazards models for drug–drug interactions, polypharmacy, and inappropriate medication use are shown in Table 2. Participants who had the potential of major drug–drug interactions relative to those with no interactions had significantly poorer survival (43% increased mortality risk). Also, those participants with a moderate drug interaction potential had a marginally significant poorer survival. Participants who used more than four medications relative to those using one medication showed a 54% decreased survival rate. Inappropriate medication use was not significantly associated with increased mortality risk.
Drug–Drug Interactions
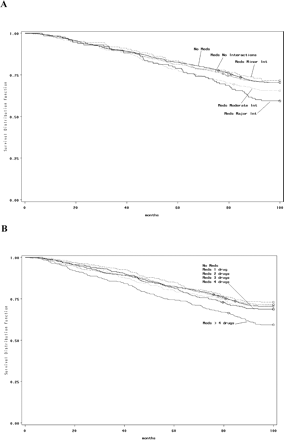
Figure 1A depicts survival by medication interaction group. Significant differences in mortality were found over time in those participants who had the potential for a major drug–drug interaction only (p <.05).
Table 3 shows the results of the adjusted and unadjusted proportional hazards model where a dichotomous drug interaction variable (major or moderate = 1; other = 0) was used to predict mortality. Relative to those with no drug interactions, those participants with a major or moderate drug interaction demonstrated a 27% increase in mortality risk (Model 1 in top part of Table 3). After adjusting for age and gender (Model 2), the increased risk remained significant. However, after adjustment for comorbid disease (Model 3) and functional status (Model 4), the increased risk for participants with a major or moderate drug interaction was no longer significant. This finding indicates that the association between drug interaction and mortality is explained by the significant comorbid factors.
Polypharmacy
Figure 1B depicts survival in the cohort by five polypharmacy groups (use of 1, 2, 3, 4, or >4 medications). Those participants taking more than four medications had significantly higher mortality rates (p <.0002).
Table 3 also shows the adjusted and unadjusted proportional hazards model in which a dichotomous polypharmacy variable is used as a predictor of mortality (>4 drugs vs ≤4 drugs). In the unadjusted model (Model 1), the risk of mortality was increased by 51% among those taking more than four medications compared to those taking four or fewer. After adjustment for demographics, comorbid illness, and functional limitations (Models 2–4), the risk of mortality associated with polypharmacy decreased to 27% but remained statistically significant.
The interaction between time and the aforementioned suboptimal medication use was not statistically significant (p =.40), demonstrating that the assumptions of proportional hazards were not violated.
Discussion
To our knowledge, this is the first study to examine the relationship of polypharmacy, drug–drug interactions, and inappropriate medication use with mortality in a community-based cohort of Hispanic older adults in the United States. Polypharmacy was a predictor of mortality, independent of age, socioeconomic status, or chronic disease status and/or severity. It has been thought that polypharmacy is potentially harmful because, in large part, it increases the probability of adverse drug–drug interactions (26,27). Our results do not appear to support this conclusion because polypharmacy alone (not potential adverse drug–drug interactions) was associated with mortality. It has been reported that adverse drug events seem to be most commonly due to failure in proper dosing (28). Rather than a direct toxicity due to adverse drug–drug interactions, polypharmacy might have a direct effect through the cumulative effects of multiple medications on the renal or hepatic systems of older adults, and cause the initiation of a “cascade of interactions” in these older adults, who already suffer from multiple comorbidities (29). Alternatively, polypharmacy increases the odds that the addition of any single medication to a frail older adult's regimen will cause a potentially adverse reaction in that individual patient. Our results are of particular importance given the mounting pressure for “recommended” polypharmacy—that is, current disease-specific treatment guidelines that are uniformly being recommended as indications of “quality” care. The preponderance of evidence supporting guidelines was primarily developed in younger participant populations without multiple comorbidities. Whether the risk of “recommended polypharmacy” outweighs the benefit is not known. This study helps to provide some understanding about the potential risk of polypharmacy. Finally, the polypharmacy rate in Mexican Americans may be lower than that expected for non-Hispanic whites because Mexican Americans use fewer over-the-counter medications (7).
Potential “inappropriate” medication use did not appear to have a mortality effect. Beer's criteria clearly state that many of the medications that fit the criteria for inappropriateness may, in fact, be indicated in certain situations. Higashi and colleagues (30) found that, among an ambulatory older adult population, pharmacologic management problems other than inappropriate medication prescribing were more common and potentially more important. Our results would seem to support the argument that medication monitoring, documentation, and continuity may be more important parameters to monitor in older populations than reduction of inappropriate medication use alone would be. More research is clearly needed to more fully delineate these issues.
Interestingly, potential major or moderate drug–drug interactions did not independently predict mortality in our participant population. Perhaps significant drug–drug interactions increase morbidity and subsequent detection leading to discontinuation of the offending medications before the drug combinations become lethal. An alternative explanation may be that the potential for a drug–drug interaction does not equal an actual interaction, and actual events may not occur frequently enough to establish a relationship between potential drug–drug interaction and mortality. Further study is needed to better understand the role of adverse drug–drug interactions in morbidity and mortality of older adults.
It is also possible that increasing comorbidity and/or disease severity leads to polypharmacy and adverse drug interactions and that the primary mortality risk might be the severity of illness and not polypharmacy or increased medication use. However, polypharmacy remained a significant independent predictor of mortality even when ADL dependency and poor self-reported health, standard proxies for illness severity, were added to our model. A limitation of this study is the self-reported nature of the interviews. We did not determine medication compliance. Furthermore, it is possible that Mexican American elders might be differentially predisposed to polypharmacy-related mortality when compared to the general population, but this predisposition would have to be an effect exclusive of demographic factors. We have previously found that Mexican American older adults taking inappropriate medications tended to fit the criteria for high vulnerability: unmarried, high physician utilization, depressed, and Medicare/Medicaid recipients (31). Another limitation was that underutilization was not examined, which may in itself be an independent predictor of mortality. Finally, although the Micromedex system is a widely used drug–drug interaction clinical tool, to our knowledge, it has not been tested for the validity or reliability of the drug–drug interaction information provided.
Despite these limitations, polypharmacy was the key suboptimal medication independent predictor for mortality in the cohort of Mexican American older adults studied. Our results indicate that increasing the number of medications alone may pose a long-term mortality risk, at least in the older Mexican American population. Further research is needed to confirm our findings.
Charles P. Mouton is now with the Department of Community Health and Family Practice, Howard University College of Medicine, Washington, DC.
Toni P. Miles is now with the Department of Family and Geriatric Medicine, University of Louisville, Louisville, Kentucky.
Decision Editor: Luigi Ferrucci, MD, PhD
Adjusted Kaplan–Meyer survival curves exploring the association between (A) potential drug–drug interactions, (B) polypharmacy, and risk of all-cause mortality during the follow-up period
Sample Characteristics Predictive of Medication Use in the Hispanic EPESE Study (N = 3050).
| Demographics . | Medication Use N = 1823 . | No Medication Use N = 1227 . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 72.98 (6.46) | 73.03 (7.17) | .840 | |||
| Mean educational level completed | 4.97 (3.96) | 4.66 (3.80) | .030 | |||
| % Female | 64.51 | 47.43 | .001 | |||
| % Poor/fair health | 72.03 | 39.13 | .001 | |||
| Annual income | ||||||
| <$10,000 | 56.62 | 58.60 | ||||
| ≥$10,000 < $20,000 | 35.38 | 34.03 | ||||
| ≥$20,000 | 8.00 | 7.37 | .570 | |||
| Cardiovascular disease | 12.46 | 4.72 | .001 | |||
| % Neoplasms | 6.28 | 3.92 | .005 | |||
| % Stroke | 7.13 | 5.00 | .017 | |||
| % Diabetes | 32.90 | 10.06 | .001 | |||
| % Hypertension | 56.79 | 19.80 | .001 | |||
| % ADL limitation | 14.49 | 12.63 | .001 | |||
| % IADL limitation | 41.49 | 54.54 | .001 | |||
| Demographics . | Medication Use N = 1823 . | No Medication Use N = 1227 . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 72.98 (6.46) | 73.03 (7.17) | .840 | |||
| Mean educational level completed | 4.97 (3.96) | 4.66 (3.80) | .030 | |||
| % Female | 64.51 | 47.43 | .001 | |||
| % Poor/fair health | 72.03 | 39.13 | .001 | |||
| Annual income | ||||||
| <$10,000 | 56.62 | 58.60 | ||||
| ≥$10,000 < $20,000 | 35.38 | 34.03 | ||||
| ≥$20,000 | 8.00 | 7.37 | .570 | |||
| Cardiovascular disease | 12.46 | 4.72 | .001 | |||
| % Neoplasms | 6.28 | 3.92 | .005 | |||
| % Stroke | 7.13 | 5.00 | .017 | |||
| % Diabetes | 32.90 | 10.06 | .001 | |||
| % Hypertension | 56.79 | 19.80 | .001 | |||
| % ADL limitation | 14.49 | 12.63 | .001 | |||
| % IADL limitation | 41.49 | 54.54 | .001 | |||
Note: EPESE = Established Populations for Epidemiologic Study of the Elderly; SD = standard deviation; ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living.
Sample Characteristics Predictive of Medication Use in the Hispanic EPESE Study (N = 3050).
| Demographics . | Medication Use N = 1823 . | No Medication Use N = 1227 . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 72.98 (6.46) | 73.03 (7.17) | .840 | |||
| Mean educational level completed | 4.97 (3.96) | 4.66 (3.80) | .030 | |||
| % Female | 64.51 | 47.43 | .001 | |||
| % Poor/fair health | 72.03 | 39.13 | .001 | |||
| Annual income | ||||||
| <$10,000 | 56.62 | 58.60 | ||||
| ≥$10,000 < $20,000 | 35.38 | 34.03 | ||||
| ≥$20,000 | 8.00 | 7.37 | .570 | |||
| Cardiovascular disease | 12.46 | 4.72 | .001 | |||
| % Neoplasms | 6.28 | 3.92 | .005 | |||
| % Stroke | 7.13 | 5.00 | .017 | |||
| % Diabetes | 32.90 | 10.06 | .001 | |||
| % Hypertension | 56.79 | 19.80 | .001 | |||
| % ADL limitation | 14.49 | 12.63 | .001 | |||
| % IADL limitation | 41.49 | 54.54 | .001 | |||
| Demographics . | Medication Use N = 1823 . | No Medication Use N = 1227 . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 72.98 (6.46) | 73.03 (7.17) | .840 | |||
| Mean educational level completed | 4.97 (3.96) | 4.66 (3.80) | .030 | |||
| % Female | 64.51 | 47.43 | .001 | |||
| % Poor/fair health | 72.03 | 39.13 | .001 | |||
| Annual income | ||||||
| <$10,000 | 56.62 | 58.60 | ||||
| ≥$10,000 < $20,000 | 35.38 | 34.03 | ||||
| ≥$20,000 | 8.00 | 7.37 | .570 | |||
| Cardiovascular disease | 12.46 | 4.72 | .001 | |||
| % Neoplasms | 6.28 | 3.92 | .005 | |||
| % Stroke | 7.13 | 5.00 | .017 | |||
| % Diabetes | 32.90 | 10.06 | .001 | |||
| % Hypertension | 56.79 | 19.80 | .001 | |||
| % ADL limitation | 14.49 | 12.63 | .001 | |||
| % IADL limitation | 41.49 | 54.54 | .001 | |||
Note: EPESE = Established Populations for Epidemiologic Study of the Elderly; SD = standard deviation; ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living.
Coefficients of the Proportional Hazards Model.
| Variable . | Estimate . | (SE) . | Hazard Ratio . | (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| No meds (n = 1227) | −.01 | (.08) | 0.99 | (0.86–1.15) | ||||
| No interaction (n = 1136) | Reference | — | — | — | ||||
| Minor interaction (n = 147) | −.06 | (.17) | 0.94 | (0.68–1.31) | ||||
| Moderate interaction (n = 412) | .19 | (.10)* | 1.21 | (0.99–1.47) | ||||
| Major interaction (n = 128) | .36 | (.15)** | 1.43 | (1.07–1.92) | ||||
| Polypharmacy | ||||||||
| No meds (n = 1227) | .03 | (.10) | 1.03 | (0.85–1.25) | ||||
| 1 med (n = 475) | Reference | — | — | — | ||||
| 2 meds (n = 398) | −.08 | (.13) | 0.92 | (0.72–1.19) | ||||
| 3 meds (n = 306) | .04 | (.14) | 1.04 | (0.80–1.36) | ||||
| 4 meds (n = 260) | .10 | (.14) | 1.11 | (0.84–1.46) | ||||
| >4 meds (n = 384) | .43 | (.12)§ | 1.54 | (1.22–1.94) | ||||
| Inappropriate medications | ||||||||
| No meds (n = 1227) | −.06 | (.07) | 0.94 | (0.82–1.08) | ||||
| Inappropriate med use (n = 414) | .06 | (.10) | 1.05 | (0.87–1.28) | ||||
| No inappropriate med use (n = 1410) | Reference | — | — | — | ||||
| Variable . | Estimate . | (SE) . | Hazard Ratio . | (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| No meds (n = 1227) | −.01 | (.08) | 0.99 | (0.86–1.15) | ||||
| No interaction (n = 1136) | Reference | — | — | — | ||||
| Minor interaction (n = 147) | −.06 | (.17) | 0.94 | (0.68–1.31) | ||||
| Moderate interaction (n = 412) | .19 | (.10)* | 1.21 | (0.99–1.47) | ||||
| Major interaction (n = 128) | .36 | (.15)** | 1.43 | (1.07–1.92) | ||||
| Polypharmacy | ||||||||
| No meds (n = 1227) | .03 | (.10) | 1.03 | (0.85–1.25) | ||||
| 1 med (n = 475) | Reference | — | — | — | ||||
| 2 meds (n = 398) | −.08 | (.13) | 0.92 | (0.72–1.19) | ||||
| 3 meds (n = 306) | .04 | (.14) | 1.04 | (0.80–1.36) | ||||
| 4 meds (n = 260) | .10 | (.14) | 1.11 | (0.84–1.46) | ||||
| >4 meds (n = 384) | .43 | (.12)§ | 1.54 | (1.22–1.94) | ||||
| Inappropriate medications | ||||||||
| No meds (n = 1227) | −.06 | (.07) | 0.94 | (0.82–1.08) | ||||
| Inappropriate med use (n = 414) | .06 | (.10) | 1.05 | (0.87–1.28) | ||||
| No inappropriate med use (n = 1410) | Reference | — | — | — | ||||
Note: *p <.10; †p <.05; ‡p <.01; §p <.001.
SE = standard error; CI = confidence interval.
Coefficients of the Proportional Hazards Model.
| Variable . | Estimate . | (SE) . | Hazard Ratio . | (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| No meds (n = 1227) | −.01 | (.08) | 0.99 | (0.86–1.15) | ||||
| No interaction (n = 1136) | Reference | — | — | — | ||||
| Minor interaction (n = 147) | −.06 | (.17) | 0.94 | (0.68–1.31) | ||||
| Moderate interaction (n = 412) | .19 | (.10)* | 1.21 | (0.99–1.47) | ||||
| Major interaction (n = 128) | .36 | (.15)** | 1.43 | (1.07–1.92) | ||||
| Polypharmacy | ||||||||
| No meds (n = 1227) | .03 | (.10) | 1.03 | (0.85–1.25) | ||||
| 1 med (n = 475) | Reference | — | — | — | ||||
| 2 meds (n = 398) | −.08 | (.13) | 0.92 | (0.72–1.19) | ||||
| 3 meds (n = 306) | .04 | (.14) | 1.04 | (0.80–1.36) | ||||
| 4 meds (n = 260) | .10 | (.14) | 1.11 | (0.84–1.46) | ||||
| >4 meds (n = 384) | .43 | (.12)§ | 1.54 | (1.22–1.94) | ||||
| Inappropriate medications | ||||||||
| No meds (n = 1227) | −.06 | (.07) | 0.94 | (0.82–1.08) | ||||
| Inappropriate med use (n = 414) | .06 | (.10) | 1.05 | (0.87–1.28) | ||||
| No inappropriate med use (n = 1410) | Reference | — | — | — | ||||
| Variable . | Estimate . | (SE) . | Hazard Ratio . | (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| No meds (n = 1227) | −.01 | (.08) | 0.99 | (0.86–1.15) | ||||
| No interaction (n = 1136) | Reference | — | — | — | ||||
| Minor interaction (n = 147) | −.06 | (.17) | 0.94 | (0.68–1.31) | ||||
| Moderate interaction (n = 412) | .19 | (.10)* | 1.21 | (0.99–1.47) | ||||
| Major interaction (n = 128) | .36 | (.15)** | 1.43 | (1.07–1.92) | ||||
| Polypharmacy | ||||||||
| No meds (n = 1227) | .03 | (.10) | 1.03 | (0.85–1.25) | ||||
| 1 med (n = 475) | Reference | — | — | — | ||||
| 2 meds (n = 398) | −.08 | (.13) | 0.92 | (0.72–1.19) | ||||
| 3 meds (n = 306) | .04 | (.14) | 1.04 | (0.80–1.36) | ||||
| 4 meds (n = 260) | .10 | (.14) | 1.11 | (0.84–1.46) | ||||
| >4 meds (n = 384) | .43 | (.12)§ | 1.54 | (1.22–1.94) | ||||
| Inappropriate medications | ||||||||
| No meds (n = 1227) | −.06 | (.07) | 0.94 | (0.82–1.08) | ||||
| Inappropriate med use (n = 414) | .06 | (.10) | 1.05 | (0.87–1.28) | ||||
| No inappropriate med use (n = 1410) | Reference | — | — | — | ||||
Note: *p <.10; †p <.05; ‡p <.01; §p <.001.
SE = standard error; CI = confidence interval.
Adjusted Proportional Hazards Model: Drug Interaction, Polypharmacy, and Mortality.
| Variable . | Model 1 Hazard Ratio (95% CI) . | Model 2 Hazard Ratio (95% CI) . | Model 3 Hazard Ratio (95% CI) . | Model 4 Hazard Ratio (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| Major or moderate interaction | 1.27 (1.08–1.49) | 1.30 (1.11–1.53) | 1.00 (0.84–1.199) | 1.04 (0.86–1.26) | ||||
| Age | — | 1.09 (1.08–1.10) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.66 (0.58–0.75) | 0.63 (0.55–0.73) | 0.57 (0.48–0.66) | ||||
| Educational level | — | 1.00 (0.98–1.02) | — | — | ||||
| Cancer | 1.89 (1.472–2.42) | 1.904 (1.43–2.53) | ||||||
| Cardiovascular disease | 1.27 (1.029–1.57) | 1.27 (1.03–1.57) | ||||||
| Diabetes | 1.75 (1.50–2.05) | 1.66 (1.39–1.97) | ||||||
| Hypertension | 1.19 (1.03–1.37) | 1.11 (0.94–1.30) | ||||||
| Stroke | 1.31 (1.04–1.66) | 1.07 (.801–1.44) | ||||||
| ADL disability | 1.13 (1.07–1.19) | |||||||
| Self-perceived health | 1.38 (1.25–1.53) | |||||||
| Polypharmacy | ||||||||
| Polypharmacy >4 | 1.51 (1.28–1.80) | 1.30 (1.11–1.53) | 1.24 (1.03–1.51) | 1.27 (1.04–1.56) | ||||
| Age | — | 1.08 (1.07–1.09) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.65 (0.57–0.74) | 0.62 (0.54–0.72) | 0.56 (0.48–0.65) | ||||
| Educational level | — | 0.99 (0.97–1.02) | — | — | ||||
| Cancer | 1.87 (1.46–2.39) | 1.86 (1.40–2.47) | ||||||
| Cardiovascular disease | 1.21 (0.97–1.49) | 0.96 (0.75–1.22) | ||||||
| Diabetes | 1.71 (1.46–2.00) | 1.63 (1.33–1.94) | ||||||
| Hypertension | 1.17 (1.01–1.35) | 1.09 (0.93–1.29) | ||||||
| Stroke | 1.30 (1.04–1.65) | 1.31 (0.79–1.41) | ||||||
| ADL disability | 1.12 (1.07–1.18) | |||||||
| Self-perceived health | 1.36 (1.24–1.51) | |||||||
| Variable . | Model 1 Hazard Ratio (95% CI) . | Model 2 Hazard Ratio (95% CI) . | Model 3 Hazard Ratio (95% CI) . | Model 4 Hazard Ratio (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| Major or moderate interaction | 1.27 (1.08–1.49) | 1.30 (1.11–1.53) | 1.00 (0.84–1.199) | 1.04 (0.86–1.26) | ||||
| Age | — | 1.09 (1.08–1.10) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.66 (0.58–0.75) | 0.63 (0.55–0.73) | 0.57 (0.48–0.66) | ||||
| Educational level | — | 1.00 (0.98–1.02) | — | — | ||||
| Cancer | 1.89 (1.472–2.42) | 1.904 (1.43–2.53) | ||||||
| Cardiovascular disease | 1.27 (1.029–1.57) | 1.27 (1.03–1.57) | ||||||
| Diabetes | 1.75 (1.50–2.05) | 1.66 (1.39–1.97) | ||||||
| Hypertension | 1.19 (1.03–1.37) | 1.11 (0.94–1.30) | ||||||
| Stroke | 1.31 (1.04–1.66) | 1.07 (.801–1.44) | ||||||
| ADL disability | 1.13 (1.07–1.19) | |||||||
| Self-perceived health | 1.38 (1.25–1.53) | |||||||
| Polypharmacy | ||||||||
| Polypharmacy >4 | 1.51 (1.28–1.80) | 1.30 (1.11–1.53) | 1.24 (1.03–1.51) | 1.27 (1.04–1.56) | ||||
| Age | — | 1.08 (1.07–1.09) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.65 (0.57–0.74) | 0.62 (0.54–0.72) | 0.56 (0.48–0.65) | ||||
| Educational level | — | 0.99 (0.97–1.02) | — | — | ||||
| Cancer | 1.87 (1.46–2.39) | 1.86 (1.40–2.47) | ||||||
| Cardiovascular disease | 1.21 (0.97–1.49) | 0.96 (0.75–1.22) | ||||||
| Diabetes | 1.71 (1.46–2.00) | 1.63 (1.33–1.94) | ||||||
| Hypertension | 1.17 (1.01–1.35) | 1.09 (0.93–1.29) | ||||||
| Stroke | 1.30 (1.04–1.65) | 1.31 (0.79–1.41) | ||||||
| ADL disability | 1.12 (1.07–1.18) | |||||||
| Self-perceived health | 1.36 (1.24–1.51) | |||||||
Note: CI = confidence interval; ADL = Activities of Daily Living.
Adjusted Proportional Hazards Model: Drug Interaction, Polypharmacy, and Mortality.
| Variable . | Model 1 Hazard Ratio (95% CI) . | Model 2 Hazard Ratio (95% CI) . | Model 3 Hazard Ratio (95% CI) . | Model 4 Hazard Ratio (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| Major or moderate interaction | 1.27 (1.08–1.49) | 1.30 (1.11–1.53) | 1.00 (0.84–1.199) | 1.04 (0.86–1.26) | ||||
| Age | — | 1.09 (1.08–1.10) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.66 (0.58–0.75) | 0.63 (0.55–0.73) | 0.57 (0.48–0.66) | ||||
| Educational level | — | 1.00 (0.98–1.02) | — | — | ||||
| Cancer | 1.89 (1.472–2.42) | 1.904 (1.43–2.53) | ||||||
| Cardiovascular disease | 1.27 (1.029–1.57) | 1.27 (1.03–1.57) | ||||||
| Diabetes | 1.75 (1.50–2.05) | 1.66 (1.39–1.97) | ||||||
| Hypertension | 1.19 (1.03–1.37) | 1.11 (0.94–1.30) | ||||||
| Stroke | 1.31 (1.04–1.66) | 1.07 (.801–1.44) | ||||||
| ADL disability | 1.13 (1.07–1.19) | |||||||
| Self-perceived health | 1.38 (1.25–1.53) | |||||||
| Polypharmacy | ||||||||
| Polypharmacy >4 | 1.51 (1.28–1.80) | 1.30 (1.11–1.53) | 1.24 (1.03–1.51) | 1.27 (1.04–1.56) | ||||
| Age | — | 1.08 (1.07–1.09) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.65 (0.57–0.74) | 0.62 (0.54–0.72) | 0.56 (0.48–0.65) | ||||
| Educational level | — | 0.99 (0.97–1.02) | — | — | ||||
| Cancer | 1.87 (1.46–2.39) | 1.86 (1.40–2.47) | ||||||
| Cardiovascular disease | 1.21 (0.97–1.49) | 0.96 (0.75–1.22) | ||||||
| Diabetes | 1.71 (1.46–2.00) | 1.63 (1.33–1.94) | ||||||
| Hypertension | 1.17 (1.01–1.35) | 1.09 (0.93–1.29) | ||||||
| Stroke | 1.30 (1.04–1.65) | 1.31 (0.79–1.41) | ||||||
| ADL disability | 1.12 (1.07–1.18) | |||||||
| Self-perceived health | 1.36 (1.24–1.51) | |||||||
| Variable . | Model 1 Hazard Ratio (95% CI) . | Model 2 Hazard Ratio (95% CI) . | Model 3 Hazard Ratio (95% CI) . | Model 4 Hazard Ratio (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Drug interaction | ||||||||
| Major or moderate interaction | 1.27 (1.08–1.49) | 1.30 (1.11–1.53) | 1.00 (0.84–1.199) | 1.04 (0.86–1.26) | ||||
| Age | — | 1.09 (1.08–1.10) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.66 (0.58–0.75) | 0.63 (0.55–0.73) | 0.57 (0.48–0.66) | ||||
| Educational level | — | 1.00 (0.98–1.02) | — | — | ||||
| Cancer | 1.89 (1.472–2.42) | 1.904 (1.43–2.53) | ||||||
| Cardiovascular disease | 1.27 (1.029–1.57) | 1.27 (1.03–1.57) | ||||||
| Diabetes | 1.75 (1.50–2.05) | 1.66 (1.39–1.97) | ||||||
| Hypertension | 1.19 (1.03–1.37) | 1.11 (0.94–1.30) | ||||||
| Stroke | 1.31 (1.04–1.66) | 1.07 (.801–1.44) | ||||||
| ADL disability | 1.13 (1.07–1.19) | |||||||
| Self-perceived health | 1.38 (1.25–1.53) | |||||||
| Polypharmacy | ||||||||
| Polypharmacy >4 | 1.51 (1.28–1.80) | 1.30 (1.11–1.53) | 1.24 (1.03–1.51) | 1.27 (1.04–1.56) | ||||
| Age | — | 1.08 (1.07–1.09) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | ||||
| Female gender | — | 0.65 (0.57–0.74) | 0.62 (0.54–0.72) | 0.56 (0.48–0.65) | ||||
| Educational level | — | 0.99 (0.97–1.02) | — | — | ||||
| Cancer | 1.87 (1.46–2.39) | 1.86 (1.40–2.47) | ||||||
| Cardiovascular disease | 1.21 (0.97–1.49) | 0.96 (0.75–1.22) | ||||||
| Diabetes | 1.71 (1.46–2.00) | 1.63 (1.33–1.94) | ||||||
| Hypertension | 1.17 (1.01–1.35) | 1.09 (0.93–1.29) | ||||||
| Stroke | 1.30 (1.04–1.65) | 1.31 (0.79–1.41) | ||||||
| ADL disability | 1.12 (1.07–1.18) | |||||||
| Self-perceived health | 1.36 (1.24–1.51) | |||||||
Note: CI = confidence interval; ADL = Activities of Daily Living.
This work was supported by Center for Medicare and Medicaid Services Grant 91467 (Medication Analysis in Mexican American Aged) and National Institute on Aging Grant 1-R01-AG10939 (Mexican American Established Populations for Epidemiologic Studies in the Elderly [EPESE]).
We thank the research support team, which includes Jennifer Acosta and Jenna Becho. We especially thank E. Mikaila Adams for her technical assistance in the preparation of this manuscript.
References
Priority Areas for National Action:. Transforming Health Care Quality, 2003. Available at: http://www.nap.edu/openbook/0309085438/html/76.html. Accessed December 7, 2003.
Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients.
Phillips DP, Christenfeld N, Glynn LM. Increase in US medication-error deaths between 1983 and 1993.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies.
Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care.
Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model.
Espino DV, Lichtnstein MJ, Hazuda HP, et al. Correlates of prescription and over-the-counter medication usage among older Mexican Americans: The Hispanic EPESE Study.
Montamat SC, Cusack B. Overcoming problems with polypharmacy and drug misuse in the elderly.
Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria determining inappropriate medication use in nursing home residents.
Flaherty JH, Perry HM, III, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients.
Golden AG, Preston RA, Barnett SD, et al. Inappropriate medication prescribing in homebound older adults.
Langdorf MI, Fox JC, Marwah RS. Physician versus computer knowledge of potential drug interactions in the emergency department.
Zhan C, Sangl J, Bierman AS, et al. Potentially inappropriate medication use in the community-dwelling elderly.
Black SA, Ray LA, Angel RJ, et al. Resource Book of the Hispanic Established Populations for Epidemiologic Studies of the Elderly. Ann Arbor, MI: National Archive for Computerized Data on Aging; 2003:1–5.
Bjerrum L, Sogaard J, Hallas J, et al. Polypharmacy: correlations with sex, age and drug regimen: a prescription database study.
Martin I, Hall J, Gardner T. Prescribing for patients aged 65 years and over in New Zealand general practice.
Kennerfalk A, Ruigomez A, Wallander MA, et al. Geriatric drug therapy and healthcare utilization in the United Kingdom.
Drug-Reax System., In: Gelman CR, Rumack BH, eds. Micromedex Intranet Knowledge Bases. Denver, CO: Micromedex, Inc; 2003.
Hazuda HP, Stern MP, Haffner SM. Acculturation and assimilation among Mexican Americans: scales and population-based data.
Fillenbaum GG. Screening the elderly: a brief instrumental activities of daily living measure.
SAS Institute Inc. SAS/STAT User's Guide. Version 6, 4th Ed., Vol. 1. Cary, NC: SAS Institute Inc; 1989.
Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley and Sons; 1980.
Monane M, Monane S, Semla T. Optimal medication use in elders. Key to successful aging.
Tanaka E. Clinically significant pharmacokinetic drug interactions between antiepileptic drugs.
Phillips J, Beam S, Brinker A, et al. Retrospective analysis of mortalities associated with medication errors.
Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients.