-
PDF
- Split View
-
Views
-
Cite
Cite
Christine R. Kovach, Yavuz Taneli, Paul Dohearty, Andrea Matovina Schlidt, Susan Cashin, Amy L. Silva-Smith, Effect of the BACE Intervention on Agitation of People With Dementia, The Gerontologist, Volume 44, Issue 6, December 2004, Pages 797–806, https://doi.org/10.1093/geront/44.6.797
Close - Share Icon Share
Abstract
Purpose: This study tests the effectiveness of the theoretically driven BACE (i.e., Balancing Arousal Controls Excesses) intervention in decreasing agitation in residents of long-term care with moderate or severe dementia. Design and Methods: A pretest–posttest double-blinded experimental design with random assignment was used with a sample of 78 participants. The BACE intervention controls the daily activity schedule so that there is a balance between a person's high-arousal and low-arousal states. The outcome measure was observed agitation. Results: When time spent in arousal imbalance at pretest was controlled for, a repeated measures analysis of covariance revealed a statistically significant Group × Time interaction, F(1, 69) = 4.26, p =.043, with a partial η2 =.06. The average change in agitation for the treatment group was a decrease of 8.43 points (SD = 12.01) from pretest to posttest, an effect size of.7. Implications: The results of this study support the theory that balancing arousal states by using an individualized approach is effective in decreasing agitation levels of people with dementia.
As many as 40–90% of people with moderate to severe dementia exhibit clinically significant agitated behaviors (Burgio, 1996; Davis, 1997). Agitation refers to excessive motor or verbal activity that is usually nonpurposeful and associated with internal tension and perceptual or cognitive abnormalities (Gordon, 1999). Agitated behaviors contribute to functional decline, increased falls and injury, social isolation, and the use of physical and chemical restraints (Beck et al., 2002; Kovach, 2000).
A growing body of research has demonstrated that agitated behaviors are not an inevitable consequence of dementia, but are often the result of external factors that can and should be controlled (Algase et al., 1996; Cohen-Mansfield, Werner, & Marx, 1990; Nelson, 1995). The Model of Imbalance in Sensoristasis (MIS) suggests that agitated behaviors may be initiated or exacerbated when there is an imbalance between sensory-stimulating and sensory-calming activity. For example, spending 4 hr in the morning in a crowded activity room doing one stimulating activity after another, or, conversely, spending 4 hr hours alone in a room with little external stimulation, would exacerbate agitation. In this study we describe daytime arousal states and test the effectiveness of the theoretically driven BACE (i.e., Balancing Arousal Controls Excesses) intervention in decreasing agitation in residents of long-term care with moderate or severe dementia (Kovach, 2000).
Background
Several models have explained behaviors commonly seen in people with dementia. The models are not mutually exclusive and are not bound to a particular setting, and factors from the four perspectives may be interactive, additive, or both (Cohen-Mansfield, 2000). The four categories of models are as follows. The first is pathophysiological: Neurological deterioration causes behavioral disinhibition (Russo-Neustadt & Cotman, 1997; Sweet et al., 1997). The second is behavioral: Behavior is controlled by triggering situations and the feedback given from others once the behavior is initiated (Teri & Logsdon, 2000; Teri, Logsdon, & McCurry, 2002). The third is unmet needs: Dementia causes an inability to comprehend or make needs known, so behaviors are mechanisms for communicating physical or psychic distress resulting from an unmet need (Algase et al., 1996). The fourth is environmental vulnerability: Dementia decreases the threshold for stress or stimuli from the environment (Hall & Buckwalter, 1987; Lawton, 1986).
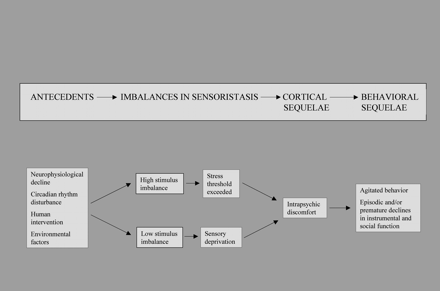
The MIS includes components from the physiological, behavioral, and unmet need models, but it focuses primarily on the person's susceptibility to environmental stress (see Figure 1). We define sensoristasis as equilibrium of the sensory state of the person with dementia. The MIS postulates that both high-arousal states and disengaged states that are sustained for a period of time create an imbalance in sensoristasis (Kovach, 2000). A long period in a state of high arousal exceeds the person's stress threshold, and a long period of disengagement creates a condition of sensory deprivation.
The MIS framework suggests that balancing time periods of high arousal and low arousal throughout the day will decrease agitation, ameliorate other behavioral symptoms, and prevent functional decline. People with significant dementia have a decreased insight into what triggers a particular emotion as well as a decreased ability to control their environment (Zeisel & Raia, 2000). According to the MIS, the person who remains for a prolonged period of time in a high-arousal state would be prescribed either some quiet rest time, daytime napping, or a lower level of activity. The person who remains for a prolonged period of time in a disengaged state would be prescribed a higher level of sensory-stimulating activity during that period of the day.
Two pilot studies support development and testing of the BACE intervention. In a descriptive study conducted with people with moderate and late-stage dementia in long-term care, Kovach and Schlidt (2001) examined differences in agitation when the same level of activity was sustained for 1.5 hr and when the level of activity was altered. They found a significant difference in agitation (t = 6.94, p <.001) between times when the same level of activity was sustained for 1. 5 hr or longer (M = 61.87, SD = 43.22) and times when level of activity was sustained for less than 1.5 hr (M = 27.20, SD = 38.08). In a similar study conducted in acute care, controlling for noxiousness of activity, Kovach and Wells (2002) found that 4.6% of the variance in agitation was uniquely accounted for by the state of activity being sustained for 1.5 hr or longer (p <.001).
An examination of the long-term-care literature revealed that one group has designed a special care unit based on an ecological model of stimulation and retreat (Lawton, van Haitsma, & Klapper, 1994). Although recognizing the need for a balance between stimulation and retreat, the intervention used at this facility represented a “global paradigmatic shift” involving the key elements of environmental changes, interdisciplinary staff training, and interdisciplinary care planning (p. S138). Care approaches were designed to create a fit between the person's compromised ability to tolerate stress in the environment and the demands of the surrounding environment.
Other research and anecdotal reports of care interventions focus on the two poles of stimulation and retreat while failing to account for the need for an individualized balance between higher and lower arousal states. Complicating matters further, many interventions can have both stimulating and calming effects and can be used to ameliorate both disengaged and high-arousal needs. Examples include music therapy (Clark, Lipe, & Bilbrey, 1998), massage (Rowe & Alfred, 1999), multisensory therapy (Threadgold, 1995), natural environments (Whall et al., 1997), and pet therapy (Hope, 1996). The stimulating or calming effect of the same intervention given in the same “dose” may vary by person, time of day, or where the person is in the course of an illness trajectory. In addition to the individual variability in response to interventions, the stimulating or calming effect may also vary based on how the interventions are designed and delivered. Although several articles provide comprehensive reviews of empirical knowledge and clinical practice regarding stimulating and calming therapeutic interventions for people with dementia (Burgener & Twigg, 2002; Hope, 1996; Peatfield, Futrell, & Cox, 2002; Pulsford, 1997), research to date has examined neither which interventions have the potential to be both stimulating and calming nor the degree or frequency of such responses.
In summary, the literature acknowledges that people with dementia need protection from both overstimulation and sensory deprivation. Empirical research is lacking, however, to establish that interventions designed to create a balance between high- and low-arousal states yield positive outcomes. Accordingly, our purpose in this study is to test the effectiveness of the BACE intervention in decreasing agitation.
Methods
Participants
Participants in this study were from 13 long-term-care facilities in the Midwest. We obtained consent for participation from both the durable power of attorney or closest family member and the participant. Of 219 persons for whom proxy consent was obtained, 10 refused consent or assent. To target the intervention to those most likely to respond and to form a homogeneous group for generalizing findings, we used eligibility criteria as follows: have a Mini-Mental State Examination (MMSE) score of 15 or below (Folstein, Folstein, & McHugh, 1975); have a Functional Assessment Staging Tool (FAST) at Stage 6 or 7 (Reisburg, Ferris, & Franssen, 1985); be identified by a nurse as having some agitation, no chronic psychiatric diagnosis other than dementia, and not currently receiving treatment for acute illness; reside at the nursing home for at least 4 weeks; and routinely sleep for less than 4 hr during the period from 7:00 a.m. to 8:00 p.m. as reported by nursing staff.
Because our interest is in studying people who have moved beyond the early stages of dementia, we used a score of 15 and below on the MMSE and Stage 6 or 7 of the FAST to categorized people who were in the later stages of dementia. The rationale for omitting people with no agitation and high levels of sleep were both ethical and practical. People that sleep for extended periods of the day and have dementia are frequently physically quite ill and may be at the end of their lives. The risk-to-benefit ratio of the BACE intervention did not justify including this group in the study. Furthermore, some people with dementia do not express overt agitation. They may be people who are less affected by the environment or who express symptoms of stress or unmet need by means of passive behaviors. We could not assess this subgroup with the agitation outcome measure. Of the 209 persons providing consent, 107 did not meet eligibility criteria (49 had no agitation; 22 had MMSE scores > 15; 21 slept > 4 hr/day; 10 had little arousal imbalance; 5 had an excluding diagnosis).
A clinically meaningful effect size for the outcome variables was considered half of the standard deviation in our earlier work. A sample of 100 (50 per group) was needed to obtain power of.8 with an effect size of.50 at a.05 alpha level. During the time period allotted for this study, 102 people participated in the study. Twelve died, 10 were dropped from the study for not being given the intervention, and 2 were excluded for erroneous or missing data. The final sample was 78, with 36 receiving the experimental intervention and 42 in the control group. The lower than expected final sample was enough to detect an effect size of.7.
Seven participants in the study were male and 71 were female. The mean length of stay was 30.2 months (SD = 25.0, range = 1–97 months). The median MMSE score for the participants was 3.00 (range 0–15); 29 participants scored zero. The mean age of the participants was 86.57 years (SD = 6.179; range 70–99). Comparisons between the experimental and control groups are shown in Table 1. The two groups were not significantly different on the variables of gender, FAST scores, age, MMSE scores, length of stay (LOS), and pretest agitation scores. The experimental group participants spent significantly more time in arousal imbalance at pretest.
Design
We used a pretest–posttest double-blinded experimental design with random assignment to groups to test differences between the experimental and control groups on the outcome variable of agitation. We randomly assigned participants to study groups in time blocks of 1 week in order to eliminate the possibility that activity of a participant in one group would influence activity of a participant in another group. We also randomized time blocks as treatment or control condition weeks.
Because there was a need to control for the influence of amount and type of activities on the outcome variables, there was a need for further control of activity between groups than offered by random assignment alone. To ensure that comparisons taken between pretest and posttest days were not confounded by the situation in which a participant experienced a stressful event at one measurement time and a typical day at another measurement, we did not collect data on days in which potentially negative or uncommon events occurred, such as on bath days, monthly doctor visit days, weekends (when staffing is often decreased or different staff work), or days in which a new or noxious exam or treatment was scheduled. We also counted the number of therapeutic recreation sessions and used this as a covariate in the analysis. Data collectors and subjects were blinded to treatment and control conditions.
We made no attempt to alter the activity schedule for control persons. During pretesting and posttesting, we collected data on the following variables: arousal state, number of therapeutic activity sessions, and agitation.
The BACE Intervention
The BACE intervention controls the daily activity schedule so that there is a balance between the time a person is in a high-arousal and a low-arousal state. The BACE intervention was initially designed to reconfigure the daily activity schedule so there was no arousal imbalance. We defined arousal imbalance as a daytime awake arousal state that was sustained for 1.5 hr or longer without change. We describe modifications to the intervention under the Intervention Integrity subsection. We chose the time period of 1.5 hr on the basis of two previous studies that supported the idea that ≥1.5 hr of high or low activity by the person with dementia was the period of time associated with the highest agitation scores (Kovach & Schlidt, 2001; Kovach & Wells, 2002).
The BACE intervention consists of three phases. Phase 1 is to make an assessment; Phase 2 is to diagnose and plan a correction of the arousal imbalance; phase 3 is to implement a new activity schedule.
During Phase 1, research assistants (RAs) measured arousal and agitation every 15 min from 8:00 a.m. to 8:00 p.m. on one day. In order to target the intervention to participants who had a substantial imbalance, we implemented Phases 2 and 3 only for those participants with ≥2.5 hr of arousal imbalance. During Phase 2, (usually the following day), information collected during Phase 1 was used by a geriatric nurse practitioner (GNP) to diagnose periods of arousal imbalance. The GNP worked with the staff nurse to configure a new daily activity schedule that (a) contained no or fewer periods of arousal imbalance and (b) was feasible, considering that person's individual needs and preferences. During Phase 3 (usually the day after Phase 2, but always within 7 days of Phase 1), the nursing staff implemented the new activity schedule and the RAs again assessed the person's state of arousal and agitation every 15 min for 12 hr.
Intervention Integrity
In some cases there was a need to add or delete some activity in order to alter the person's activity schedule. However, adding or deleting activities could threaten the validity of the BACE intervention by contaminating the intervention with therapeutic activity interventions. We used two procedures to control this threat. First, the BACE intervention focused on preserving, to the extent possible, the same activities as on Day 1 data collection, but altered the timing of some activities to eliminate or decrease arousal imbalance. Second, data were collected on the number of therapeutic activity sessions per day so that this variable could be used as a covariate in the analysis.
Early in the study, we made attempts to eliminate all arousal imbalances, but it quickly became apparent that in many cases it was neither realistic nor therapeutic to induce this many changes in the activity schedule of the participant. For example, some participants had high arousal states throughout the day. It was not possible to make such a drastic reduction in their activity schedule over such a short time period. Therefore, we attempted to correct the imbalance as much as the nurse judged feasible.
A participant had to have been given at least one of the prescribed changes in activity schedule, regardless of whether the person responded to the prescribed activity schedule change, to be included in the analysis. For example, if one additional rest or quiet period in the morning and one additional arousing activity in the afternoon were ordered, at least one of these changes had to be made. Nurses stated the intervention was not given in 10 cases because of being short-staffed, being busy, or having more emergent needs the day of the scheduled intervention. Before a person was dropped from the study for not being given any dose of intervention, two coders not associated with the project independently confirmed that the person had been given no intervention.
Another potential ethical concern was that a participant could have so many scheduled therapeutic treatments that, to operationalize the intervention, therapies would have to be eliminated. This situation did not arise, but it was another criterion for dropping participants to avoid possibly compromising curative or rehabilitative efforts.
Measurement
The MMSE is a widely used instrument that measures short-term and long-term memory, orientation, attention, calculation, registration, language, praxis, and copying of a design (Folstein et al., 1975). The MMSE has demonstrated both reliability and validity, with test scores correlating with age-adjusted scores on the Wechsler Adult Intelligence Scale (Zarit, 1997). The FAST is a 16-item simple checklist that divides function into seven stages (Reisburg et al., 1985). Stages 6 and 7 represent the lowest functional levels. A Guttman analysis revealed a coefficient of scalability of.98 and a coefficient of reproducibility of.99, supporting the undimensional and cumulative qualities of the scale. Concurrent validity of FAST Stages 6 and 7 with the Ordinal Scales of Psychological Development was supported by a correlation of −.79. Intraclass correlation coefficients to assess interrater reliability were.86 for rater consistency and.87 for rater agreement (Sclan & Reisberg, 1992).
This study relies on the direct observation of participants for the measurement of arousal, activities, and agitation. Observational techniques, although time intensive, are superior to the commonly used retrospective recall of behaviors. Retrospective recall has resulted in memory bias and other measurement errors. Alertness level of people with dementia has been reliably measured through electroencephalography, but the use of this testing equipment would have compromised study conditions (Edman, Brunovsky, Sjogren, Wallin, & Matousek, 2003).
Observations occurred from 8:00 a.m. to 8:00 p.m. on a pretesting (Phase 1 of intervention) and posttesting (Phase 3 of intervention) day. Participants were observed for 3 min every 15 min. Three participants were observed per day, and a random order table was used to vary the sequence of observing those three participants throughout the day. Observations did not take place in the bathroom, if the door was closed to the bedroom, or if the bedside privacy curtain was closed.
Prior to data collection, RAs underwent a thorough training program to familiarize themselves with observational methodologies in general, and with the arousal and agitation tools in particular. Training at sites continued until interrater reliability estimates were.85 or higher for all observational tools on five sequential measures. We conducted interrater reliability checks every 2–3 months and we retrained data collectors anytime reliability dropped below.80. Training continued until interrater reliability was.85. Following training sessions, interrater reliabilities were between.88 and 1.0 for arousal and ranged from.95 to.984 for agitation.
Arousal and Activity
Arousal was observed and ranked by use of the Arousal States in Dementia (ASD) Scale (Table 2). The ASD Scale was developed by the principal investigator. Arousal is defined as the participant's state of stimulation to action. The most aroused state observed in the 3-min time period was marked on a data-collection form. To obtain consistency when differentiating minimal and high arousal, we made sure the training included use of a goniometer to familiarize data collectors with degrees of limb and body movement. For example, exercise class would be considered high arousal, whereas socializing could be minimal or high arousal, depending on degree of limb movement. In addition to ranking arousal states, we provided space on the form so that collectors could briefly describe what the person was doing, or what was being done to the person (e.g., eating a meal, or sitting at a table and looking around). We used these descriptions of activity to plan the intervention and to identify that a person had been given the intervention.
Arousal Balance and Imbalance
We examined 12 hr of arousal data collection for each participant. We considered arousal imbalance to be 1.5 hr or more time spent at the same level of arousal. When the same arousal state was sustained for <1.5 hr, we considered it a time of arousal balance. We calculated the amount of time spent in arousal balance and imbalance to provide arousal balance and imbalance scores.
Agitation
Agitation is recognized as a sensitive outcome for people with acute and chronic neurological disorders, including those causing irreversible dementia (Gordon, 1999). Our initial plan called for us to assess agitation by using the Agitation Behavior Mapping Instrument (ABMI; Cohen-Mansfield et al., 1989). We were, however, unable to achieve sufficiently consistent interrater reliability estimates during training, particularly when a participant's behavior was not at the extremes. We then trained RAs to assess agitation by using a visual analog scale from 0 to 100. We defined agitation as excessive motor, vocal, or verbal activity that is usually nonpurposeful and associated with internal tension and perceptual or cognitive abnormalities (Gordon, 1999). The behavior is not explained by the events (i.e., need of the situation), and in a nondemented person in a similar situation, the behavior would not be expected (Cohen-Mansfield et al., 1989). In addition, we operationalized agitation according to the 29 items of the Cohen-Mansfield Agitation Inventory (Cohen-Mansfield, 1999).
RAs marked the visual analog scale by utilizing the agitation intensity parameters shown in Table 3. The maximum score for each line was 100. For example, if a respondent displayed two minimally intense behaviors (e.g., move repetitively for 10 s and scratch for 40 s) then the score would be a 75, because the two behaviors lasted 50 s. If the person bit another person for 10 s then the score would be 100, because this is a high level of motor activity that is not usual.
Therapeutic Activity
We defined therapeutic activities as staff-elicited activities that last at least 10 min, were individual or group oriented, implemented to meet therapeutic goals, and involved recreation that stimulated the senses. Examples include social visiting, exercise, fine-motor skill recreation activity, music listening, storytelling, and reminiscence.
Results
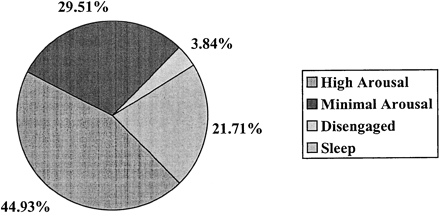
We examined pretest scores to provide a general description of agitation and arousal state. As seen in Figure 2, the participants of this sample spent about half of the day in the high level of arousal, averaging 5.38 hr (SD = 2.72) of the 12-hr daytime data collection. Minimal arousal states occurred on average for 3.53 hr (SD = 3.25), and participants slept an average of 2.6 hr (SD = 2.25). Disengaged states were least frequent, occurring on average 27.6 min/12 hr (SD = 56.93). Thirteen subjects spent 8 hr or more in the highest aroused state, and, of those, 6 spent 10 hr or more in a state of high arousal.
Pretest agitation scores ranged from 1.16 to 91.46, with the average agitation of 35.53 (SD = 21.26). There were no significant correlations between agitation and the amount of time spent in high arousal (r =.105, p =.363), minimal arousal (r =.051, p =.665), or disengaged (r = −.115, p =.320) states.
Participants averaged 6.27 hr (SD = 2.69) of arousal imbalance/12 hr at pretest. Twenty-one participants spent 8 hr or more in a state of arousal imbalance, and, of those, 9 spent 10 hr or more in a state of arousal imbalance during the 12 hr of data collection. At pretest, respondents had significantly more agitation during periods of arousal imbalance (M = 38.72, SD = 24.43) than during periods of arousal balance (M = 24.38, SD = 22.73); t = 4.65, p <.001. There was a statistically significant difference between the treatment and control groups in time spent in arousal imbalance at pretest (t = 2.57, p <.01), with the experimental group averaging 7.16 hr (SD = 2.5) and the control group averaging 5.64 hr (SD = 2.7). Because random assignment did not equalize the groups on arousal imbalance, we controlled for this variable in the analysis.
Table 4 presents the repeated measures analysis of covariance (ANCOVA) results comparing pretest and posttest agitation scores between the BACE intervention and control groups. To adjust for differences in arousal imbalance and MMSE at pretest, we entered these variables into the model as covariates. There was a statistically significant Group × Time interaction, F(1, 69) = 4.26, p =.046, with a partial η2 =.06. We added the number of therapeutic activities given at posttest to the model as a second covariate, but this was not a statistically significant variable.
The mean agitation score for the intervention group at posttest was 30.54 (SD = 15.31), and for the control group it was 32.25 (SD = 20.16). We conducted a stratified analysis to examine changes in agitation scores for the treatment and control groups. Whereas the mean agitation score for the control group stayed the same from pretest to posttest, the mean in the intervention group changed from 38.97 (SD = 20.54) to 30.54 (SD = 15.31). This was a significant change in agitation for the BACE intervention group: t(35) = 4.21, p <.001. The average change in agitation was a decrease of 8.43 points (SD = 12.01) from pretest to posttest, an effect size of.7. For the control group the paired difference was not significant; t(41) = −.308, p =.760. An examination of frequency distributions revealed that 30 of the 36 respondents in the treatment group had a decrease in agitation from pretesting to posttesting. Decreases in agitation ranged from 1.91 points to 30.55 points. Fourteen respondents decreased their agitation score by 10 or more points, and 6 respondents decreased it by 25 or more points.
In order to determine if the intervention was changing agitation scores more during arousal imbalance than arousal balance times, we performed paired t-test comparisons for agitation from pretest to posttest during arousal balance and imbalance (see Table 5). For each participant, we summed and averaged agitation scores collected every 15 min (excluding times of sleep) to calculate an agitation during arousal imbalance and an agitation during arousal balance score. Agitation scores decreased significantly from pretesting to posttesting during the arousal imbalance time periods for the BACE intervention group (t = 2.13, p =.04). Although the mean agitation score also decreased during imbalanced arousal states, the 2.54-point decrease did not reach a level of statistical significance (t =.554, p =.583).
In order to determine if differences in agitation between the BACE intervention group and the control group were dependent on other factors, we calculated three separate 2 × 2 repeated measure ANCOVAs. The three models included the pretest arousal imbalance as a covariate and posttest agitation as the dependent variable. The interaction variable in Model 1 was a MMSE score dichotomized as severely impaired (i.e., MMSE ≤ 9) and moderately impaired (i.e., MMSE ≥ 10). The interaction variable in Model 2 was the amount of time spent sleeping over the 12 hr, dichotomized into whether the person slept a lot during the day (i.e., >2 hr/12 hr) or a little (i.e., ≤2 hr). The interaction variable in Model 3 was whether the person had lost or retained verbal skills (as indicated in Substage 7a or 7b of the FAST). There were no significant interaction effects in the three repeated measure ANCOVAs run using the three models, indicating that differences in agitation between the BACE intervention group and the control group were not dependent on whether the person was moderately or severely cognitively impaired [F(1, 73) =.485, p =.488], whether the person slept a lot or a little during the day [F(1, 72) =.092, p =.762], or whether the person had retained or lost verbal skills [F(1, 72) =.043, p =.836].
We defined dose as the percentage of the activity schedule ordered that was actually given. Participants were prescribed between one and four changes in activity schedule, with 72% of the treatment group being prescribed one or two activity changes and 28% being prescribed three or four changes. The full dose of the prescribed activity schedule change was given to 72% of the sample, whereas 22% received 50–75% of the prescribed changes and 6% received less than 50% of the prescribed dose of activity schedule changes. Regression analyses revealed that dose was not significantly associated with postagitation scores (B = −.101, p =.426).
Discussion
An examination of the pretest data in this study revealed that the persons in the sample spent the majority of their day in engaged aroused states. In addition, agitation was significantly higher during times of arousal imbalance than during times of arousal balance. These results are consistent with data from our two earlier studies (Kovach & Schlidt, 2001; Kovach & Wells, 2002). It is interesting that participants in this study and our earlier study in long-term care spent only a small amount of the day in a disengaged state (Kovach & Schlidt, 2001). These empirical findings are inconsistent with popular notions of people with significant dementia as being largely disengaged from the environment and activities in the environment.
The results of this study support the concept that balancing time spent in higher and lower arousal states through manipulation of activity schedules is effective in decreasing agitation levels of people with dementia. In this study, differences in agitation between the BACE intervention group and the control group were not dependent on cognitive level, verbal ability, or amount of time spent sleeping during the day. The standard deviation of the agitation scores from pretest to posttest also decreased in the intervention group. The effect size of the intervention was moderate and suggests that the BACE intervention is effective for decreasing agitation and improving quality of life for those most severely affected by dementia. This intervention is simple, low cost, noninvasive, and nontechnological.
There is consensus in the literature that behaviors associated with dementia are a complex issue, with multiple causal factors coming into play. The sampling plan in this study targeted the intervention to people with significant dementia who experienced at least some agitation and were awake for a large portion of the day. However, for 6 participants, the BACE intervention did not decrease agitation. These individuals may not be as affected by imbalances in arousal states, may not have been given or received a high enough dose of the intervention, or had other factors contributing to their agitation.
This study utilized a tailored intervention in which, rather than utilizing a standardized treatment, the intervention was customized to the individual's specific need. The trend toward tailored research was spurred, in part, by low effect sizes in intervention research and the disparities between health and psychosocial care as practiced and as studied (Lauver et al., 2002). This type of research acknowledges individual needs and differences, and it is more consistent with the delivery of holistic care. Methodologically, there is a need to disentangle the separate and combined effects of the arousal imbalance correction versus the mode of delivering changes in activity schedule.
Examining the influence of dose through linear regression analysis did not reveal an association between dose of BACE intervention delivered and agitation. This may indicate that dose did not matter or that dose response is nonlinear. Agitation was a short-term outcome measure. Dose may affect other outcomes or may affect longer term measures. Future research should examine factors that affect dose and predict responsiveness to the intervention such as differential tolerance of imbalances in sensoristasis between individuals and within individuals based on time of day, station along the illness trajectory, or the type of illness.
A large number of consenting participants did not meet eligibility criteria. This study is generalizable only to people who are similar to the participants in this study. Of particular interest are the 49 people who were dropped from the study for no or rare agitation. We used nurses' reports of agitation, and this may have led us to exclude people who had agitated behavior that was less obvious or less troublesome to the nurses. This group may include people who exhibit withdrawn or apathetic responses to unmet needs but would have benefited from the intervention. The Model of Imbalance in Sensoristasis addresses the need for a balance in sensory stimulation. This study involved balancing the level of arousal by altering the person's daily activity schedule. Future research is needed that more directly tests the model through specific measurement of sensory stimulation and more long-term outcomes such as function and behavior over time. Future research should also examine the influence of balancing activity during the daytime on nighttime sleep and agitation.
In order to prevent treatment carryover, we randomly assigned participants to study groups in time blocks of 1 week. This study did not measure if activity of a participant in the treatment group influenced activity of a participant in the control group. If spillover from treatment to control persons did occur, it would have decreased the magnitude of difference between groups. In addition, although we were able to control for therapeutic activities as a confounder, because the control group received no intervention and the treatment group did, the differences in agitation could have been due to changes in quality of activity or interactions that occurred normally in the setting. This study relied on random assignment to equalize the medication use of the groups. Because we did not measure psychotropic medication use, if differences were present, this could have affected differences between the groups.
The multidimensional nature of agitation and other behaviors associated with dementia is widely acknowledged. Literature in the past decade has evolved from considering these behaviors as caregiving problems to a broader recognition of the underlying need and distress these behaviors represent. The results of this study have the potential to change practice by offering a new intervention for the treatment of agitation. Use of the BACE intervention may also be effective in preventing agitation. Future research should examine this preventive capacity as well as the effect of the intervention on additional behavioral outcomes and physiological responses. Evidence from studies of the adult mammalian cortex suggests that nonthreatening sensory stimulation enhances synaptic connections (Zito & Svoboda, 2002), suggesting the need for examination of neurological as well as other physiological effects. There is also a need to refine understanding of the factors that predict need for the BACE intervention, tolerance for imbalances in sensoristasis, and optimum dosing of the intervention.
Funding from the Helen Bader Foundation supported this project. We thank Dr. Thelma Wells, RN, PhD, FAAN, FRCN, Professor Emerita and Sheryl Kelber, MS for their thoughtful review of this manuscript.
College of Nursing, University of Wisconsin–Milwaukee.
School of Architecture and Urban Planning, University of Wisconsin–Milwaukee.
Department of Sociology, University of Wisconsin–Milwaukee.
Statistician, University of Wisconsin–Milwaukee.
Decision Editor: Linda S. Noelker, PhD
Model of imbalance in sensoristasis. Figure is reprinted with the permission of Christine R. Kovach
Percentage of time spent in arousal states (measures taken at pretest; n = 78)
Description of Participants Experimental and Control Group.
| . | Group . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Experimental (n=36) . | Control (n=42) . | Test Statistic . | Probability p . | ||||
| Gender | ||||||||
| Male | 5 | 2 | χ2 = 1.977 | .16 | ||||
| Female | 31 | 40 | ||||||
| FAST: function | ||||||||
| Stage 6 | 19 | 16 | χ2 = 3.806 | .149 | ||||
| Stage 7 | 16 | 26 | ||||||
| Age | ||||||||
| M | 86.81 | 86.34 | t = −.295 | .769 | ||||
| SD | 6.411 | 6.041 | ||||||
| MMSE: cognition | ||||||||
| M | 5.63 | 3.66 | t = −1.692 | .095 | ||||
| SD | 5.117 | 4.811 | ||||||
| Length of stay | ||||||||
| M | 32.44 | 28.56 | t = −.703 | .484 | ||||
| SD | 24.229 | 23.230 | ||||||
| Arousal imbalance, min/12 hr | ||||||||
| M | 107.4 | 84.6 | t = −2.584 | .012* | ||||
| SD | 37.50 | 40.50 | ||||||
| Agitation | ||||||||
| M | 38.97 | 32.58 | t = −1.334 | .186 | ||||
| SD | 20.54 | 21.66 | ||||||
| . | Group . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Experimental (n=36) . | Control (n=42) . | Test Statistic . | Probability p . | ||||
| Gender | ||||||||
| Male | 5 | 2 | χ2 = 1.977 | .16 | ||||
| Female | 31 | 40 | ||||||
| FAST: function | ||||||||
| Stage 6 | 19 | 16 | χ2 = 3.806 | .149 | ||||
| Stage 7 | 16 | 26 | ||||||
| Age | ||||||||
| M | 86.81 | 86.34 | t = −.295 | .769 | ||||
| SD | 6.411 | 6.041 | ||||||
| MMSE: cognition | ||||||||
| M | 5.63 | 3.66 | t = −1.692 | .095 | ||||
| SD | 5.117 | 4.811 | ||||||
| Length of stay | ||||||||
| M | 32.44 | 28.56 | t = −.703 | .484 | ||||
| SD | 24.229 | 23.230 | ||||||
| Arousal imbalance, min/12 hr | ||||||||
| M | 107.4 | 84.6 | t = −2.584 | .012* | ||||
| SD | 37.50 | 40.50 | ||||||
| Agitation | ||||||||
| M | 38.97 | 32.58 | t = −1.334 | .186 | ||||
| SD | 20.54 | 21.66 | ||||||
*Statistically significant at the.05 alpha level.
Note: MMSE = Mini-Mental State Examination; FAST = Functional Assessment Staging Tool.
Description of Participants Experimental and Control Group.
| . | Group . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Experimental (n=36) . | Control (n=42) . | Test Statistic . | Probability p . | ||||
| Gender | ||||||||
| Male | 5 | 2 | χ2 = 1.977 | .16 | ||||
| Female | 31 | 40 | ||||||
| FAST: function | ||||||||
| Stage 6 | 19 | 16 | χ2 = 3.806 | .149 | ||||
| Stage 7 | 16 | 26 | ||||||
| Age | ||||||||
| M | 86.81 | 86.34 | t = −.295 | .769 | ||||
| SD | 6.411 | 6.041 | ||||||
| MMSE: cognition | ||||||||
| M | 5.63 | 3.66 | t = −1.692 | .095 | ||||
| SD | 5.117 | 4.811 | ||||||
| Length of stay | ||||||||
| M | 32.44 | 28.56 | t = −.703 | .484 | ||||
| SD | 24.229 | 23.230 | ||||||
| Arousal imbalance, min/12 hr | ||||||||
| M | 107.4 | 84.6 | t = −2.584 | .012* | ||||
| SD | 37.50 | 40.50 | ||||||
| Agitation | ||||||||
| M | 38.97 | 32.58 | t = −1.334 | .186 | ||||
| SD | 20.54 | 21.66 | ||||||
| . | Group . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Variable . | Experimental (n=36) . | Control (n=42) . | Test Statistic . | Probability p . | ||||
| Gender | ||||||||
| Male | 5 | 2 | χ2 = 1.977 | .16 | ||||
| Female | 31 | 40 | ||||||
| FAST: function | ||||||||
| Stage 6 | 19 | 16 | χ2 = 3.806 | .149 | ||||
| Stage 7 | 16 | 26 | ||||||
| Age | ||||||||
| M | 86.81 | 86.34 | t = −.295 | .769 | ||||
| SD | 6.411 | 6.041 | ||||||
| MMSE: cognition | ||||||||
| M | 5.63 | 3.66 | t = −1.692 | .095 | ||||
| SD | 5.117 | 4.811 | ||||||
| Length of stay | ||||||||
| M | 32.44 | 28.56 | t = −.703 | .484 | ||||
| SD | 24.229 | 23.230 | ||||||
| Arousal imbalance, min/12 hr | ||||||||
| M | 107.4 | 84.6 | t = −2.584 | .012* | ||||
| SD | 37.50 | 40.50 | ||||||
| Agitation | ||||||||
| M | 38.97 | 32.58 | t = −1.334 | .186 | ||||
| SD | 20.54 | 21.66 | ||||||
*Statistically significant at the.05 alpha level.
Note: MMSE = Mini-Mental State Examination; FAST = Functional Assessment Staging Tool.
Categories for the Arousal State in Dementia Scale.
| Category . | Definition . |
|---|---|
| Sleep | Eyes are closed with no purposeful movement of the body. |
| Disengaged | Eyes are open but not focused on a particular event or person. The person may be physically mobile or immobile but there is no apparent purposeful activity. |
| Minimal Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movement of arms, legs, head, and trunk is minimal (either <20 deg or occurs for <1 min). |
| High Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movements of arms, legs, head, and trunk are more than minimal (either >20 deg or occurs for >1 min). |
| Category . | Definition . |
|---|---|
| Sleep | Eyes are closed with no purposeful movement of the body. |
| Disengaged | Eyes are open but not focused on a particular event or person. The person may be physically mobile or immobile but there is no apparent purposeful activity. |
| Minimal Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movement of arms, legs, head, and trunk is minimal (either <20 deg or occurs for <1 min). |
| High Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movements of arms, legs, head, and trunk are more than minimal (either >20 deg or occurs for >1 min). |
Categories for the Arousal State in Dementia Scale.
| Category . | Definition . |
|---|---|
| Sleep | Eyes are closed with no purposeful movement of the body. |
| Disengaged | Eyes are open but not focused on a particular event or person. The person may be physically mobile or immobile but there is no apparent purposeful activity. |
| Minimal Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movement of arms, legs, head, and trunk is minimal (either <20 deg or occurs for <1 min). |
| High Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movements of arms, legs, head, and trunk are more than minimal (either >20 deg or occurs for >1 min). |
| Category . | Definition . |
|---|---|
| Sleep | Eyes are closed with no purposeful movement of the body. |
| Disengaged | Eyes are open but not focused on a particular event or person. The person may be physically mobile or immobile but there is no apparent purposeful activity. |
| Minimal Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movement of arms, legs, head, and trunk is minimal (either <20 deg or occurs for <1 min). |
| High Arousal | Eyes are focused on events, objects, or persons in the environment. Activity is purposeful and movements of arms, legs, head, and trunk are more than minimal (either >20 deg or occurs for >1 min). |
Wisconsin Agitation Intensity Parameters.
| Score . | Behavior . | . | And–Or . | Intensity . | |
|---|---|---|---|---|---|
| Number . | Duration . | ||||
| 0 | 0 | — | — | The person was entirely calm | |
| 25 | 1 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 50 | 1 | 16–59s | Or | > Minimal motor, verbal, or vocal behavior | |
| 50 | 2 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 1 | 60–119s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 2 | 16–59s | And | Minimal motor, verbal, or vocal behavior | |
| 100 | 1 | ≥120 s | Or | High motor, verbal, or vocal behavior | |
| 100 | ≥2 | ≥60s | Or | High motor, verbal, or vocal behavior | |
| Score . | Behavior . | . | And–Or . | Intensity . | |
|---|---|---|---|---|---|
| Number . | Duration . | ||||
| 0 | 0 | — | — | The person was entirely calm | |
| 25 | 1 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 50 | 1 | 16–59s | Or | > Minimal motor, verbal, or vocal behavior | |
| 50 | 2 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 1 | 60–119s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 2 | 16–59s | And | Minimal motor, verbal, or vocal behavior | |
| 100 | 1 | ≥120 s | Or | High motor, verbal, or vocal behavior | |
| 100 | ≥2 | ≥60s | Or | High motor, verbal, or vocal behavior | |
Wisconsin Agitation Intensity Parameters.
| Score . | Behavior . | . | And–Or . | Intensity . | |
|---|---|---|---|---|---|
| Number . | Duration . | ||||
| 0 | 0 | — | — | The person was entirely calm | |
| 25 | 1 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 50 | 1 | 16–59s | Or | > Minimal motor, verbal, or vocal behavior | |
| 50 | 2 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 1 | 60–119s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 2 | 16–59s | And | Minimal motor, verbal, or vocal behavior | |
| 100 | 1 | ≥120 s | Or | High motor, verbal, or vocal behavior | |
| 100 | ≥2 | ≥60s | Or | High motor, verbal, or vocal behavior | |
| Score . | Behavior . | . | And–Or . | Intensity . | |
|---|---|---|---|---|---|
| Number . | Duration . | ||||
| 0 | 0 | — | — | The person was entirely calm | |
| 25 | 1 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 50 | 1 | 16–59s | Or | > Minimal motor, verbal, or vocal behavior | |
| 50 | 2 | ≤15 s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 1 | 60–119s | And | Minimal motor, verbal, or vocal behavior | |
| 75 | 2 | 16–59s | And | Minimal motor, verbal, or vocal behavior | |
| 100 | 1 | ≥120 s | Or | High motor, verbal, or vocal behavior | |
| 100 | ≥2 | ≥60s | Or | High motor, verbal, or vocal behavior | |
Means, Standard Deviations, and Repeated Measures ANCOVA Results for BACE Groups.
| Agitation Score . | BACE Group . | . | Control Group . | . | ||
|---|---|---|---|---|---|---|
| M . | SD . | M . | SD . | |||
| Pretest | 38.97 | 20.54 | 32.59 | 21.66 | ||
| Posttest | 30.54 | 15.31 | 32.25 | 20.16 | ||
| Agitation Score . | BACE Group . | . | Control Group . | . | ||
|---|---|---|---|---|---|---|
| M . | SD . | M . | SD . | |||
| Pretest | 38.97 | 20.54 | 32.59 | 21.66 | ||
| Posttest | 30.54 | 15.31 | 32.25 | 20.16 | ||
Notes: ANCOVA = analysis of covariance; BACE = Balancing Arousal Controls Excesses.
Partial η2 =.06; Pretest to posttest × Group, F(1, 69) = 4.26; p = 0.43.
Means, Standard Deviations, and Repeated Measures ANCOVA Results for BACE Groups.
| Agitation Score . | BACE Group . | . | Control Group . | . | ||
|---|---|---|---|---|---|---|
| M . | SD . | M . | SD . | |||
| Pretest | 38.97 | 20.54 | 32.59 | 21.66 | ||
| Posttest | 30.54 | 15.31 | 32.25 | 20.16 | ||
| Agitation Score . | BACE Group . | . | Control Group . | . | ||
|---|---|---|---|---|---|---|
| M . | SD . | M . | SD . | |||
| Pretest | 38.97 | 20.54 | 32.59 | 21.66 | ||
| Posttest | 30.54 | 15.31 | 32.25 | 20.16 | ||
Notes: ANCOVA = analysis of covariance; BACE = Balancing Arousal Controls Excesses.
Partial η2 =.06; Pretest to posttest × Group, F(1, 69) = 4.26; p = 0.43.
Differences in Agitation From Pretesting to Posttesting During Arousal Imbalance and Balance for the BACE Intervention Group.
| Variable . | Agitation (n=36) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Pretest . | Posttest . | t . | p . | ||||
| Arousal imbalance time periods | 41.68 (22.85) | 35.56 (22.00) | 2.13 | .040* | |||
| Arousal balance time periods | 27.30 (27.34) | 24.76 (21.59) | .554 | .583 | |||
| Variable . | Agitation (n=36) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Pretest . | Posttest . | t . | p . | ||||
| Arousal imbalance time periods | 41.68 (22.85) | 35.56 (22.00) | 2.13 | .040* | |||
| Arousal balance time periods | 27.30 (27.34) | 24.76 (21.59) | .554 | .583 | |||
Note: BACE = balancing arousal controls excesses.
* Statistically significant result.
Differences in Agitation From Pretesting to Posttesting During Arousal Imbalance and Balance for the BACE Intervention Group.
| Variable . | Agitation (n=36) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Pretest . | Posttest . | t . | p . | ||||
| Arousal imbalance time periods | 41.68 (22.85) | 35.56 (22.00) | 2.13 | .040* | |||
| Arousal balance time periods | 27.30 (27.34) | 24.76 (21.59) | .554 | .583 | |||
| Variable . | Agitation (n=36) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Pretest . | Posttest . | t . | p . | ||||
| Arousal imbalance time periods | 41.68 (22.85) | 35.56 (22.00) | 2.13 | .040* | |||
| Arousal balance time periods | 27.30 (27.34) | 24.76 (21.59) | .554 | .583 | |||
Note: BACE = balancing arousal controls excesses.
* Statistically significant result.
References
Algase, D. L., Beck, C., Kolanowski, A., Whall, A., Berent, S., & Richards, K., et al (
Beck, C. K., Vogelpohl, T. S., Rasin, J. H., Uriri, J. T., O'Sullivan, P., & Walls, R., et al (
Burgener, S., & Twigg, P., (
Burgio, L. D., (
Clark, M. E., Lipe, A. W., & Bilbrey, M., (
Cohen-Mansfield, J., (
Cohen-Mansfield, J., (
Cohen-Mansfield, J., Werner, P., & Marx, M. S., (
Cohen-Mansfield, J., Werner, P., & Marx, M. S., (
Davis, G. C., (
Edman, A., Brunovsky, M., Sjogren, M., Wallin, A., & Matousek, M., (
Folstein, M. F., Folstein, S. E., & McHugh, P. R., (
Gordon, V., (
Hall, G. R., & Buckwalter, K. C., (
Hope, K., (
Kovach, C. R., (
Kovach, C. R., & Schlidt, A. M., (
Kovach, C. R., & Wells, T., (
Lauver, D. R., Ward, S. E., Heidrich, S. M., Keller, M. L., Bowers, B. J., & Brennan, P. F., et al (
Lawton, M. P., Van Haitsma, K., & Klapper, J., (
Nelson, J., (
Peatfield, J. G., Futrell, M., & Cox, C. L., (
Pulsford, D., (
Reisburg, B., Ferris, S. H., & Franssen, E., (
Rowe, M., & Alfred, D., (
Russo-Neustadt, A., & Cotman, C. W., (
Sclan, S. G., & Reisberg, B. R., (
Sweet, R. A., Pollock, B. G., Mulsant, B. H., Rosen, J., Lo, K. H., & Yao, J. K., et al (
Teri, L., & Logsdon, R. G., (
Teri, L., Logsdon, R. G., & McCurry, S. M., (
Whall, A. L., Black, M. E., Groh, C. J., Yankou, D. J., Kupferschmid, B. J., & Foster, N. L., (
Zeisel, J., & Raia, P., (