-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshiaki Kikkawa, Philomena Mburu, Sue Morse, Ryo Kominami, Stuart Townsend, Steve D.M. Brown, Mutant analysis reveals whirlin as a dynamic organizer in the growing hair cell stereocilium, Human Molecular Genetics, Volume 14, Issue 3, 1 February 2005, Pages 391–400, https://doi.org/10.1093/hmg/ddi035
Close - Share Icon Share
Abstract
Little is known of the molecular processes that lead to the growth of stereocilia on the surface of hair cells in the inner ear. The PDZ protein whirlin is known, by virtue of the whirler mutation, to be involved in the process of stereocilia elongation and actin polymerization in the sensory hair cells of mammals. We have investigated the function of whirlin and its putative interacting partner, myosin XVa, in the stereocilium using relevant mice mutants. We raised an antibody that detects the short isoform of the whirlin protein which has been demonstrated to rescue the stereocilia growth defect in the whirler mutant. We show that whirlin localizes at the tips of stereocilia. Expression of whirlin is dynamic during stereocilia growth, demonstrating an ordered appearance and fade-out across the stereocilia rows and revealing a novel molecular gradation of process traversing the stereocilia bundle. Fade-out of whirlin in inner hair cells precedes that of outer hair cells, consistent with the earlier maturation of inner hair cell stereocilia. In myosin XVa mutants in which stereocilia are shortened, whirlin expression in the stereocilia tips is stalled and fade-out is accelerated. In whirlin mutants, myosin XVa is still expressed in stereocilia, but its appearance at the stereocilia tip is delayed. The data indicate that whirlin expression is a critical and dynamic organizer for stereocilia elongation and actin polymerization.
INTRODUCTION
Stereocilia, actin-filled structures on the surface of hair cells, are vital for the process of mechanotransduction in the auditory and vestibular systems. Stereocilia are organized into bundles whose extraordinary feature is a regular staircase pattern. The bundles consist of several rows of stereocilia that are ordered according to height. Stereocilia develop from microvilli on the surface of hair cells in the region of the kinocilium (1). Microvilli initially elongate, a process that begins at around E17.5 in mice. Subsequently, in mammals, stereocilia thicken and continue to elongate and during the early stages of this latter phase, the staircase pattern appears (2,3). Stereocilia closest to the kinocilium elongate first, followed by the next row and so on. Once the staircase pattern has emerged, it is maintained during subsequent growth. In mice, by P1 the staircase structure has appeared in inner hair cells and is well developed in outer hair cell rows (4). By P5–P7, the process of morphological maturation is complete. The mechanism by which the staircase pattern develops is unknown and it is not known if there are further important growth processes that continue beyond the period of obvious morphological maturation.
Stereocilia in adjacent rows are connected by tip-links (5,6) that attach the tips of shorter stereocilia to the sides of neighbouring taller stereocilia. It has been proposed that the tip-links attach at sites on the stereocilia that harbour the mechanotransduction channels such that sound stimulation would lead to stereocilia deflection, increase in tip-link tension and channel opening followed by cation influx and hair cell depolarization (7). However, it is also postulated that the linkages between stererocilia may affect the ordered growth of the staircase pattern of stereocilia (8). Just as with sound stimulation, growth of one stereocilium would increase tension on the tip-links that connect neighbouring stereocilia in the adjacent shorter row, opening the transduction channels and promoting Ca influx. If the processes of actin polymerization and stereocilia growth are enhanced by Ca influx, then a positive feedback loop would exist between adjacent stereocilia, which would tend to ensure the maintenance of the graded staircase structure and its coordinated growth.
The actin core of stereocilia is organized such that the barbed (plus) ends of actin filaments are located at the tips of stereocilia in the region of actin polymerization (9). There is a continuous cycle of renewal of actin filaments such that addition of actin monomers at the stereocilia tips occurs throughout adult hair cell life (10,11). To date, what little we know of the molecular processes that control the actin polymerization and stereocilia growth have come from the studies of mouse mutants that show defects in stereocilia development, including the shaker2 and whirler mutants, which harbour mutations in the myosin XVa (12,13) and whirlin (14) genes, respectively.
Mutations in myosin XVa lead to shortened stereocilia (13). Myosin XVa is an unconventional myosin whose expression is first detected from around E13.5 in the developing inner ear (15). Subsequently, by E18.5 it is restricted to the hair cells, where it is expressed at the tips of the growing stereocilia (16). Myosin XVa expression extends beyond the growth period of stereocilia and throughout the adult life. It appears that myosin XVa is necessary for stereocilia growth (18). The mutation in the whirlin gene also leads to a stereocilia growth defect (14,17). In whirler mutant homozygotes, inner hair cell stereocilia are considerably shorter than those of wild-type littermates. Outer hair cell stereocilia in homozygous mutants are closer to normal in length but are arranged in a rounded U-shape fashion compared with the normal V or W shape, and stereocilia at the edges of rows are shortened. Whirlin is a novel PDZ protein expressed in stereocilia, comprising three PDZ domains and a proline-rich region. At least two major isoforms are transcribed from the whirlin gene, including a long isoform and a truncated short isoform that contains only the C-terminal proline-rich region and the PDZ3 domain and lacks the two N-terminal PDZ domains. The short isoform has been demonstrated to achieve almost complete rescue of the whirler phenotype (14). The phenotype of the whirler mutant suggests that the whirlin protein acts as an organizer of submembranous complexes that control the coordinated actin polymerization and membrane growth of stereocilia (14). It has been suggested that myosin XVa and whirlin might interact through the PDZ domains of whirlin (18).
In order to investigate the function of whirlin and its associated complexes in the process of stereocilia growth, we have undertaken analysis of the expression of whirlin and other proteins in both the whirler and shaker2 mutants. Whirlin shows an unusual dynamic expression pattern traversing the stereocilia bundle, which reveals evidence of a novel molecular process underlying the process of stereocilia growth.
RESULTS
Whirlin localization in stereocilia
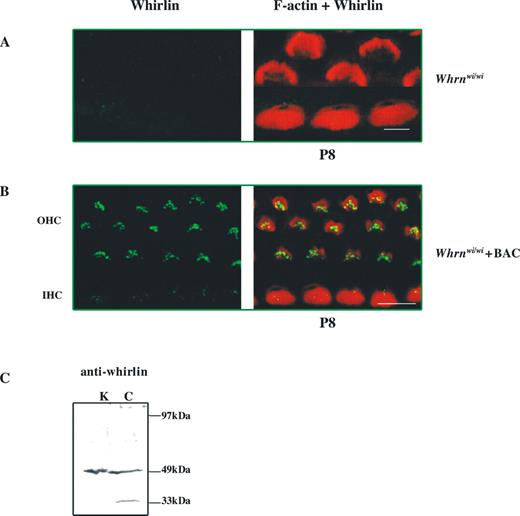
The effects of disruption of the whirlin gene indicate a role for whirlin in the process of stereocilia growth and actin polymerization. Previous work (14) had demonstrated that whirlin was localized in the stereocilia of the hair cell. We have now investigated the detailed localization of whirlin protein in the stereocilium in hair cells in both the organ of Corti and the vestibular system (Fig. 1). We generated an antibody to two peptides in the C-terminal region of the whirlin protein that is common to both short and long isoforms of whirlin [(14) and See Materials and Methods]. The whirlin antibody fails to show any labelling to cochlea of whirler homozygous (wi/wi) mice (Fig. 1A). However, labelling of both IHCs and OHCs (Fig. 1B) is seen in whirler homozygous mice carrying a BAC transgene expressing the short whirlin isoform that rescues the whirler phenotype (14). These experiments confirm the specificity of the antibody and that it is able to detect the critical whirlin isoform responsible for rescuing the stereocilia growth defect (see Introduction). Interestingly, the level of expression of the short isoform is higher in OHCs than in IHCs. We had already shown that N-terminal antibodies to the whirlin gene, which detect the long isoform but not the short isoform, detect higher levels of expression in the IHCs when compared with the OHCs (14). In contrast, C-terminal antibodies generated previously (14), which were presumed to detect both long and short isoforms, showed similar levels of expression in IHCs and OHCs suggesting that the short isoform is expressed at higher levels in the OHCs than in the IHCs and consistent with our observations on the expression of the short isoform in the BAC rescued mice—which contrasts with that observed for the long isoform as reported previously (14). We also carried out western analysis to explore the specificity of the antibody (Fig. 1C). We found that the antibody detected the expected 49 kDa band that corresponds to the short isoform in both cochlea and kidney tissues (Fig. 1C). An additional band of 33 kDa was detected in the cochlea, which may represent an additional short isoform expressed in this tissue. However, the antibody failed to detect any longer isoforms including the established 97 kDa long isoform (14).
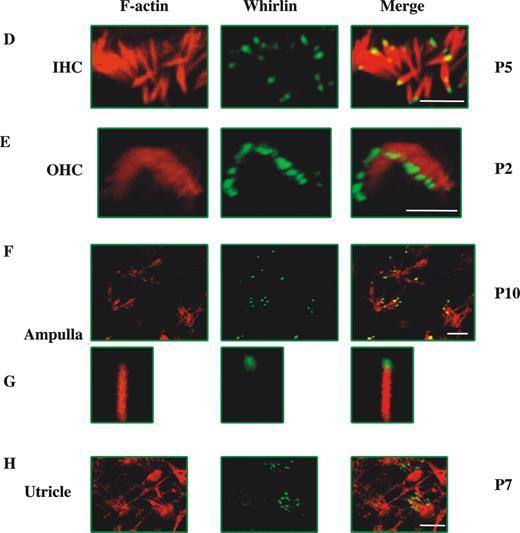
We initially utilized the antibody to investigate the localization of whirlin in IHCs, OHCs and vestibular hair cells in C3H/HeN wild-type mice at time points where the stereocilia bundle is mature (Fig. 1). We carried out dual labelling for actin and whirlin for both the organ of Corti and vestibular system whole mount preparations. The merged images indicate localization of whirlin to the relatively actin-free zone at the tips of stereocilia in both IHCs and OHCs in the organ of Corti (Fig. 1D and E) and the hair cells of the vestibular system (Fig. 1F–H) We cannot rule out some labelling that overlaps the actin core or low levels of whirlin in the shaft of stereocilia. Nevertheless, whirlin is expressed at the stereocilia tip. We thus investigated in detail the expression and localization of whirlin from P0 to P16 encompassing the period of stereocilia development and beyond.
Dynamic expression of whirlin in outer hair cells during stereocilia development
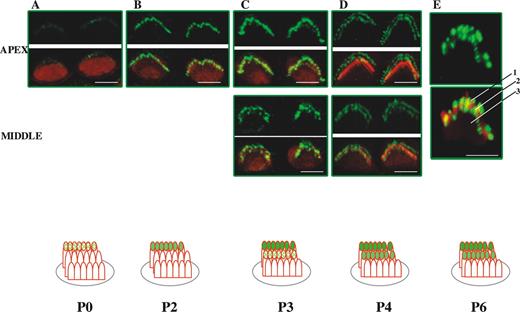
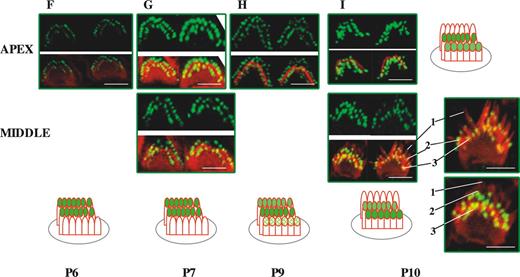
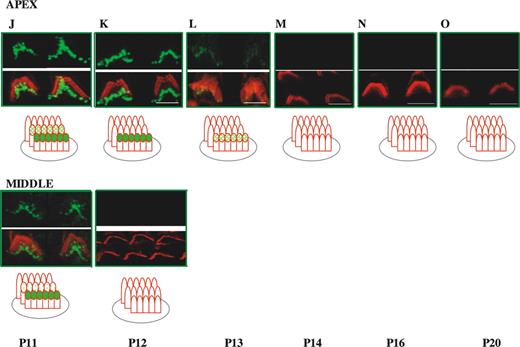
We first analyzed the expression of whirlin in OHCs for developmental stages between P0 and P20. We focused most of our studies on OHCs from the apical turn of the cochlea (Fig. 2). However, for some developmental stages, we carried out comparative labelling on OHCs from the middle turn of the cochlea that are more advanced in development. In OHCs, the staircase pattern is already developed by P1 (see Introduction). Planar views of the expression of whirlin in OHCs at early time points from the cochlear apical turn (P0–P2) reveal only one discrete row of labelled stereocilia tips corresponding to the outer row of stereocilia (Fig. 2A and B). By P3, we find that whirlin expression is beginning to emerge at stereocilia tips in the middle row of stereocilia revealed by the appearance of a second discrete row of labelled stereocilia tips (Fig. 2C). This is more apparent at P3 in preparations from the middle turn of the cochlea in which hair cell development is more advanced. By P4, there is strong labelling of the outer and middle rows of stereocilia that is sustained till P7–P8 (Fig. 2D, E and G). A more lateral view of the stereocilia bundle at P6 (Fig. 2E) confirms the presence of whirlin at the stereocilia tips of the outer and middle rows of stereocilia. At P9, there appears to be some labelling of the third inner row of stereocilia (Fig. 2H). By P10, fade-out of whirlin expression in the outer row of stereocilia has occurred and labelling is now confined to the middle and inner rows of stereocilia and sustained until P11 (Fig. 2I–K). This is confirmed by a lateral view of the stereocilia bundle from the middle turn at P10, where the outer row of stereocilia is unlabelled but whirlin is expressed at stereocilia tips in the middle and inner stereocilia rows (Fig. 2I). At P12, in apical cochlea turns there is fade-out of whirlin expression from the middle row of stereocilia such that only one discrete row of stereocilia tips now remains labelled (Fig. 2K). Fade-out of expression of whirlin from the middle row of stereocilia is already advanced at P11 in middle turns of the cochleae (Fig. 2J). In apical turns of the cochlea from P13–P14, fade-out of whirlin expression from the inner row of stereocilia occurs (Fig. 2L) and from P14 onwards no labelling of OHC stereocilia can be detected (Fig. 2M–O). As expected, fade-out of expression of whirlin from the inner row of stereocilia in the middle turns of the cochlea occurs early and is complete by P12 (Fig. 2K). We observed no detectable labelling of OHC stereocilia at later adult time points up to P20. In summary, the detailed analysis of whirlin expression reveals an unusual pattern by which whirlin shows an ordered appearance and fade-out that traverses the stereocilia rows.
Expression of whirlin in inner hair cells and vestibular hair cells during stereocilia development
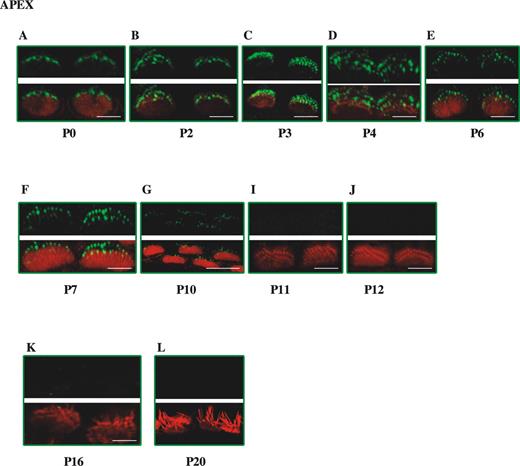
We also investigated the time course of expression of whirlin in IHCs (Fig. 3). Expression of whirlin appears at the stereocilia tips of IHCs at P0. Initially, it appears from planar views that only one discrete row of stereocilia is labelled at P0 (Fig. 3A). Subsequently from P2 onwards additional rows of stereocilia tips appear to be labelled. However, the less well organized arrangement of the stereocilia staircase in IHC bundles means that it has not been possible to determine whether there is a similar traverse of whirlin expression across the stereocilia rows to that seen in OHCs. However, fade-out of whirlin expression in IHCs occurs at P11, earlier than in OHCs.
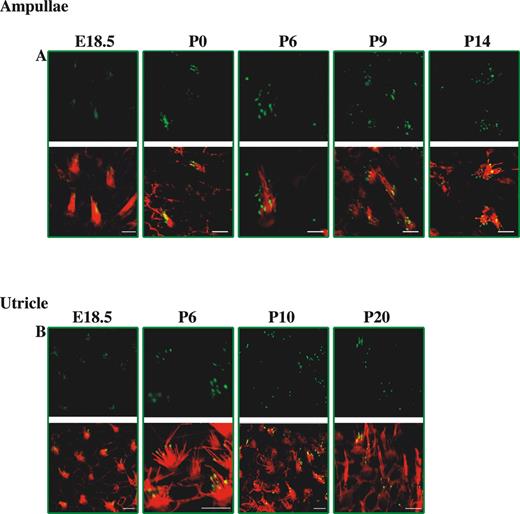
We also studied the expression of whirlin in the stereocilia of the ampullae and utricle of the vestibular system from E18.5 to adulthood (Fig. 4). Whirlin is first expressed at E18.5 in both the ampullae and the utricle (Fig. 4A and B). However, in contrast to the expression pattern of whirlin in the organ of Corti, we found no variation in the patterns of expression in the vestibular ampullae and utricle at subsequent time points. Whirlin is constitutively expressed at the stereocilia tips at all time points into adulthood.
Functional analysis of whirlin and myosin XVa in stereocilia using the whirler and shaker2 mutants
As previously reported (16), myosin XVa is localized at the tips of the stereocilia of the cochlear and vestibular hair cells. It is expressed from E18.5 at the tips of stereocilia and expression is sustained throughout adulthood. Indeed, it has been hypothesized (18) that whirlin and myosin XVa might interact mediated either through the proline-rich domain of whirlin and the SH3 domain of myosin XVa, or alternatively, via the class 1 PDZ-binding motif at the C-terminus of myosin XVa and the several PDZ domains of whirlin. Indeed, co-localization and in vitro pull-down experiments reveal that myosin XVa and whirlin do interact. It was shown that myosin XVa interacts with the short isoform of whirlin via the myosin SH3 domain and the whirlin proline-rich domain (C. Petit, personal communication and accompanying paper). Myosin XVa might therefore provide a vehicle for transporting and localizing whirlin at the tips of stereocilia. For this reason, we have explored the functional interactions of these proteins by investigating the expression of myosin XVa and whirlin in the whirler and shaker2 mutants, respectively.
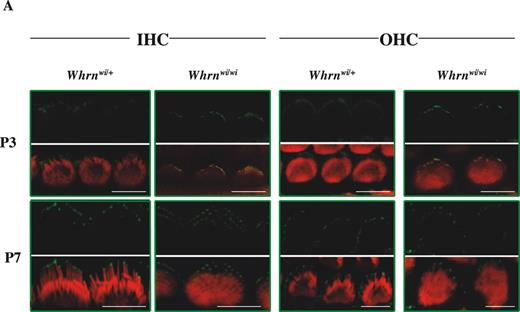
In order to investigate the functional relationship between myosin XVa and whirlin, we first examined the localization of myosin XVa in whirler (wi/wi) mutant mice. In wi/wi mutant mice, IHC stereocilia are short and stubby, whereas OHC stereocilia are more normal in shape though the usual V-shaped arrangement of stereocilia is more rounded and stereocilia at the edges of rows are shortened. We hypothesized that if myosin XVa's key role were to effect whirlin transport to the stereocilia tip, its localization would be unaffected in the whirler mutant. In homozygous whirler mutant mice, myosin XVa is still clearly localized within the stereocilia (Fig. 5A). However, it appears that localization of myosin XVa at the stereocilia tip is compromised. At P3, analysis of whirler homozygotes indicates that there is significant myosin XVa localization outside of the actin-free zone at the stereocilia tip in contrast to wild-type. However, by P7, myosin XVa is localized at the stereocilia tip and demonstrates patterns of labelling indistinguishable from wild-type.
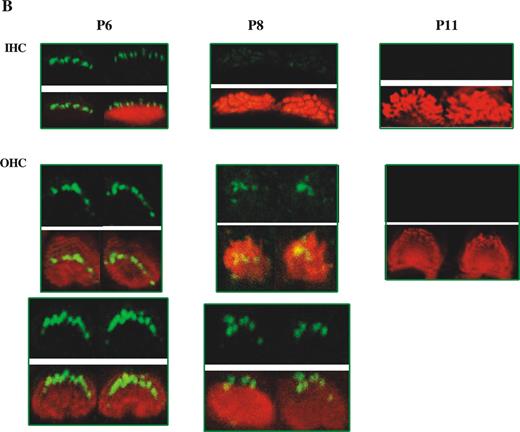
We also performed the complementary experiment and investigated the expression of whirlin in the shaker2 mutant (sh2/sh2). In sh2/sh2 mutant mice, there is no detectable myosin XVa expression (16). We found that the normal expression pattern of whirlin was disturbed in IHCs and OHCs (Fig. 5B). We observed by P6 that only one row of stereocilia was consistently labelled and that no additional rows of stereocilia were labelled as might be expected by this time point. It appears that the expression pattern of whirlin is stalled. In wild-type mice, at P6, whirlin labelling has already appeared in the second row of stereocilia (Fig. 2). Moreover, there was premature fade-out of whirlin expression so that little whirlin labelling was observed after P6 and fade-out was complete by P11. It is clear that myosin XVa plays a major role in the proper development and stabilization of whirlin expression in the stereocilia.
DISCUSSION
Whirlin, by virtue of its mutations, is known to be a key protein for stereocilia growth (14). In order to elaborate whirlin's function in actin polymerization and stereocilia growth, we have sought first to investigate its expression both across space and time during the development of the stereocilia bundle. Importantly, we firstly characterized the antibody raised to whirlin and demonstrated that it detected the short isoform of whirlin. There are a number of transcripts produced by the whirlin gene and it is possible that different whirlin isoforms will show differing expression patterns. Given that the antibody we had generated was able to detect the short isoform that rescues the whirler mutant phenotype and is clearly pivotal for proper stereocilia growth, we proceeded to examine the expression of whirlin during stereocilia development.
Whirlin shows an extraordinarily dynamic and exquisite expression pattern across the period of stereocilia growth. The bulk of whirlin expression is localized in the actin-free zone at the stereocilia tip which is the region involved in growth and remodelling of the actin core (10,11). As a PDZ protein involved in the process of stereocilia growth, this finding underlines the possibility that whirlin is involved in assembling critical complexes for actin polymerization at the barbed end region of the actin filaments at the stereocilia tip. Whirlin's expression overlaps the period of stereocilia growth as we showed that expression fades out in OHCs and IHCs at P14 and P11, respectively. We cannot rule out that there is a low undetectable level of whirlin expressed into adulthood. In addition, we cannot exclude that the expression pattern observed may also reflect changes in epitope exposure. In contrast to the cochlea, whirlin expression is maintained at high levels at the tips of stereocilia in vestibular hair cells through to adulthood. Interestingly, the expression of whirlin in IHCs overlaps the time period at which IHC stereocilia stop elongating in the whirler mutant. Developmental analysis (17) indicates that at E18.5 whirler mutant stereocilia are already significantly shorter than those of controls. However, they elongate until P1 but stop elongating prematurely between P1 and P4 and begin to regress.
Intriguingly, in OHCs, during the period of stereocilia growth, the expression of whirlin is not uniform across all the rows that make up the characteristic staircase pattern of stereocilia. Rather, whirlin accumulation at stereocilia tips begins in the tallest OHC stereocilia and makes an ordered appearance and fade-out traversing from the taller stereocilia rows to the shorter, before fading out completely. The data raise a number of questions, not least what process traffics whirlin to individual stereocilia in a strict temporal fashion. However, these observations provide, for the first time, evidence indicating that the growth status of stereocilia is not uniform, but shows a molecular gradation of process starting with the taller stereocilia and traversing to the shorter. It will be illuminating to search for ultrastructural, physiological and further molecular correlates of this molecular process. In this regard, there are several possible explanations for the dynamic traverse of whirlin expression across the stereocilia rows. It seems likely that whirlin is required at the stereocilia tip when the stereocilium has reached a key growth point. Given that whirlin is expressed at high levels beyond P5–P7 when mouse stereocilia are thought to be morphologically mature, it suggests that growth processes may be continuing beyond this point. If this were the case, then whirlin could be responsible for assembling protein complexes that might include capping proteins involved in terminating the polymerization and growth of actin fibres. However, it is not clear, given that actin fibres within the stereocilium undergo constant treadmilling both during development and in adults (11), that the process of termination of actin fibre growth that leads to stereocilia elongation will require capping proteins. Alternatively, whirlin might play a role in the establishment and maintenance of tip-link structures that connect stereocilia tips in adjacent rows and are vital for the process of auditory transduction (7). However, tip-link structures and transduction currents appear early in the development of stereocilia around E16–E17 (18). Given the expression profile of whirlin, it would seem more likely to be involved in the maintenance or stabilization of tip-links during early development, rather than the establishment of the structure. Alternatively, whirlin may be involved in transporting or assembling additional components of the tip-link associated transduction machinery once the tip-link is formed. Identification of interacting partners to the whirlin protein will assist in distinguishing the various possible roles.
Despite the similar localization of whirlin and myosin XVa at the stereocilia tip, their developmental expression is different. Myosin XVa is expressed in the stereocilia tips from around E18.5 at the time of initiation of stereocilia development and is sustained into adulthood (16). In contrast, the strongest period of expression of whirlin in OHCs and IHCs encompasses the period of stereocilia growth. It has been proposed that whirlin and myosin XVa are interacting partners, both playing key roles in stereocilia development (19), and it could be that myosin XVa plays a role in transporting whirlin to the stereocilia tip. It is unclear what vehicle may be responsible for transporting whirlin from the stereocilia tip. However, we show here that the ablation of whirlin or myosin XVa in the relevant mutants shows an effect on the localization of the corresponding protein. In whirler mutants, the localization of myosin XVa at the stereocilia tips appears compromised. In shaker2 mutants, whirlin expression appears stalled at the tips of stereocilia but fade-out of expression is accelerated. It appears that myosin XVa is required for the proper developmental expression and stabilization of whirlin at the stereocilia tips but our results would indicate that some whirlin can be trafficked to the stereocilia tip in the absence of myosin XVa. Equally, a lack of whirlin expression appears to compromise the expression of myosin XVa at the stereocilia tips in vivo.
In conclusion, the PDZ protein whirlin shows an extraordinary pattern of expression across the mouse stereocilia bundle. Along with evidence from studies employing mouse mutants, it appears that whirlin is a key and dynamic organizer at the stereocilia tip for the process of stereocilia growth and actin polymerization. Moreover, the exquisite dynamics of whirlin expression indicates the existence of a novel molecular mechanism underpinning stereocilia growth.
MATERIALS AND METHODS
Mutant mice stocks
Shaker2 mutant mice were obtained from the Jackson Laboratory (Bar Harbor, MI, USA) and a stock maintained at Harwell. Whirler mutant mice were obtained from a stock maintained at Harwell.
Antibody production
We generated rabbit immune serum against the mouse whirlin protein. We raised anti-Wct2 against a mixture of two peptides in the C-terminal end of the protein (amino acids 572–582, SPSEDLPGIKP and amino acids 598–610 GQPRKPGREDPAP). We also generated rabbit immune serum against the mouse MYO15a protein. Antibodies were raised against a mixture of two peptides LKEIQSTWTQKPTA (amino acid 2252–2265) and KLRAEQRRRAQEAWLR (amino acid 767–782).
Immunolocalization studies
The cochlear ducts of C3H/HeN, Whrnwi/wi and Myo15ash2/sh2 mice ranging in age from E18.5 to P20 were fixed in 4% paraformaldehyde in PBS for 1 h at 4°C followed by three quick washes in PBS at room temperature (RT). Fixed cochlear ducts were permeabilized in TBST (25 mm Tris, 3 mm KCl, 1.4 m NaCl and 0.01% Tween-20) buffer for 10 min, and non-specific binding sites blocked with 0.5% blocking reagent (Roche Diagnostics) for 1 h at RT. Samples were incubated with affinity purified whirlin or with MyoXVa antiserum diluted 1 : 100 and 1 : 200, respectively, in 0.5% blocking reagent, initially for 1 h at RT and then at 4°C overnight with gentle shaking. After 3×10 min washes in TBST buffer, non-specific binding sites were blocked with 0.5% blocking reagent for 30 min at RT. Samples were then incubated with anti-rabbit FITC-conjugated secondary antibody diluted 1 : 250 in 0.5% blocking reagent for 1 h at RT followed by 3×10 min washes in TBST and 2×5 min washes in PBS. F-actin was visualized by rhodamine–phalloidin staining (molecular probes) according to the manufacturer's recommendations. The samples were then mounted in PermaFlour™ aqueous mounting medium (ThermoShandon), and viewed on BioRad Radiance 2100 confocal microscope.
Immunoblot analysis
Kidneys and cochlear ducts from new born mice were homogenized in PBS containing 0.5 mm PMSF, 1 mm DTT and 0.5 mm EDTA. From each tissue, 10 µg protein were resolved on a 12% SDS–PAGE gel, transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) and subjected to immunoblot analysis. Primary antibody was rabbit anti-whirlin and the secondary antibody was horseradish peroxidase-conjugated anti-rabbit. Results were visualized using enhanced chemiluminescence (Amersham Biosciences).
ACKNOWLEDGEMENTS
We thank Karen Steel for comments on an earlier version of this manuscript. This work was supported by the Medical Research Council, UK.
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors.
Figure 1. Whirlin localizes to stereocilia tips in the hair cells of the organ of Corti and the vestibular system. (A–C) Specificity of the whirlin antibody. (A, B) Confocal images of whirlin protein localization in (A) whirler homozygous mice (Whrnwi/wi) and (B) whirler homozygous mice (Whrnwi/wi) transgenic for BAC279 that rescues the stereocilia phenotype (14) and carries the short isoform of the whirlin gene. (C) Western blot of the whirlin antibody. A band of 49 kDa corresponding to the short isoform is seen in both kidney (K) and cochlea (C). Cochlea carries an additional smaller band of 33 kDa possibly representing an additional short isoform expressed in this tissue. (D–H) Confocal images of whirlin protein localization in wild-type (C3H/HeN) mice. (D) P5 mouse inner hair cell stereocilia (apical turn of cochlea); (E) P2 mouse outer hair cell stereocilia (apical turn of cochlea); (F) P10 ampullae hair cells stereocilia (ampulla); (G) an individual enlarged stereocilium from ampullae hair cells; (H) P7 utricle hair cells. Rhodamine-phalloidin staining in the red channel highlights the actin core of stereocilia. Merged images highlight whirlin protein localization in actin-free (green) and actin-rich (yellow) areas. Scale bars=5 µm.
Figure 2. Developmental study of whirlin expression in outer hair cell stereocilia bundles in the mouse. Confocal images of whirlin protein expression in wild-type (C3H/HeN) mice OHCs from P0 to P20. Rhodamine–phalloidin staining in the red channel highlights the actin core of stereocilia. Merged images highlight whirlin protein localization in actin-free (green) and actin-rich (yellow) areas. Diagram illustrates the different localization of whirlin protein in the OHC stereocilia three rows across development (see Results). Scale bars=2 µm. The outermost, middle and innermost rows of stereocilia are labelled 1, 2 and 3, respectively.
Figure 3. Developmental study of whirlin expression in inner hair cell stereocilia bundles in the mouse. Confocal images of whirlin protein expression in wild-type (C3H/HeN) mice IHCs from P0 to P20. Rhodamine–phalloidin staining in the red channel highlights the actin core of stereocilia. Merged images highlight whirlin protein localization in actin-free (green) and actin-rich (yellow) areas. Scale bars=2 µm.
Figure 4. Developmental study of whirlin expression in vestibular hair cell stereocilia bundles in the mouse. Confocal images of whirlin protein expression in vestibular hair cells of the ampullae and utricle from E18.5 to P20 wild-type (C3H/HeN) mice. Rhodamine–phalloidin staining in the red channel highlights the actin core of stereocilia. Merged images highlight whirlin protein localization in actin-free (green) and actin-rich (yellow) areas. Scale bars=2 µm.
Figure 5. Analysis of myosin XVa and whirlin localization in the whirlin and shaker2 mutants. (A) Comparison of localization of myosin XVa (green) between wild-type (C3H/HeN) mice and whirlin-deficient mouse (Whrnwi/wi) in stereocilia of IHCs and OHCs of the organ of Corti (apical turns of cochlea) at P3 and P7. Rhodamine–phalloidin staining in the red channel highlights the actin core of stereocilia. Merged images highlight myosin XVa protein localization in actin-free (green) and actin-rich (yellow) areas. (B) Localization of whirlin (green signals) in myosin XVa-deficient mouse (Myo15ash2/sh2) stereocilia of IHCs and OHCs of the organ of Corti (apical turns of cochlea) at P6, P8 and P11. Scale bars=5 µm.
References
Tilney, L.G., Tilney, M.S. and DeRosier, D.J. (
Forge, A., Souter, M. and Denman-Johnson, K. (
Denman-Johnson, K. and Forge, A. (
Anniko, M. (
Pickles, J.O., Comis, S.D. and Osborne, M.P. (
Pickles, J.O., von Perger, M., Rouse, G.W. and Brix, J. (
Tilney, L.G., Tilney, M.S. and Cotanche, D.A. (
Tilney, L.G., Derosier, D.J. and Mulroy, M.J. (
Schneider, M.E., Belyantseva, I.A., Azevedo, R.B. and Kachar, B. (
Rzadzinka, A.K., Schneider, M.E., Davies, C., Riordan, G.P. and Kachar, B. (
Wang, A., Liang, Y., Fridell, R.A., Probst, F.J., Wilcox, E.R., Touchman, J.W., Morton, C.C., Morell, R.J., Noben‐Trauth, K., Camper, S.A. and Friedman, T.B. (
Probst, F.J., Fridell, R.A., Raphael, Y., Saunders, T.L., Wang, A., Liang, Y., Morell, R.J., Touchman, J.W., Lyons, R.H., Noben‐Trauth, K. et al. (
Mburu, P., Mustapha, M., Varela, A., Weil, D., El‐Amraoui, A., Holme, R.H., Rump, A., Hardisty, R.E., Blanchard, S., Coimbra, R.S. et al. (
Anderson, D.W., Probst, F.J., Belyantseva, I.A., Fridell, R.A., Beyer, L., Martin, D.M., Wu, D., Kachar, B., Friedman, T.B. Raphael, Y. et al. (
Belyantseva, I.A., Boger, E.T. and Friedman, T.B. (
Holme, R.H., Kiernan, B.W., Brown, S.D. and Steel, K.P. (
Geleoc, G.S. and Holt, J.R. (