-
PDF
- Split View
-
Views
-
Cite
Cite
David H. Skuse, X-linked genes and mental functioning, Human Molecular Genetics, Volume 14, Issue suppl_1, 15 April 2005, Pages R27–R32, https://doi.org/10.1093/hmg/ddi112
Close - Share Icon Share
Abstract
The X-chromosome has played a crucial role in the development of sexually selected characteristics for over 300 million years. During that time it has accumulated a disproportionate number of genes concerned with mental functions. Evidence is emerging, from studies of both humans and mice, for a general influence upon intelligence (as indicated by the large number of X-linked mental retardation syndromes). In addition, there is evidence for relatively specific effects of X-linked genes on social–cognition and emotional regulation. Sexually dimorphic processes could be influenced by several mechanisms. First, a small number of X-linked genes are apparently expressed differently in male and female brains in mouse models. Secondly, many human X-linked genes outside the X–Y pairing pseudoautosomal regions escape X-inactivation. Dosage differences in the expression of such genes (which might comprise at least 20% of the total) are likely to play an important role in male–female neural differentiation. To date, little is known about the process but clues can be gleaned from the study of X-monosomic females who are haploinsufficient for expression of all non-inactivated genes relative to 46,XX females. Finally, from studies of both X-monosomic humans (45,X) and mice (39,X), we are learning more about the influences of X-linked imprinted genes upon brain structure and function. Surprising specificity of effects has been described in both species, and identification of candidate genes cannot now be far off.
ORIGINS OF THE X-CHROMOSOME
The autosomes and the sex chromosomes differ in their evolutionary origins, a fact that may have implications for the distinct contribution made by the X-chromosome to mental functioning. There are estimated to be 931 genes on the X-chromosome (Ensembl version 26.35.1), ∼3.75% of all genes. In 2004, Online-Mendelian Inheritance in Man recorded 1237 entries for ‘mental retardation’. Of these, 333 (27%) mapped to the X-chromosome, suggesting X-linked genes play a disproportionate role in the development of human intelligence. Why should there be such a concentration on this particular chromosome (1)? Zechner et al. (2) suggest that the X-chromosome has been engaged in the development of sexually selected characteristics for at least 300 million years and that natural selection has favoured the development of X-linked genes that are associated with higher cognitive abilities. In particular, males are more likely than females to be influenced by haplotypes that are associated with exceptionally high abilities. For an equivalent reason, they are also more likely to show deficits in mental abilities than females because of the impact of deleterious mutations carried in haploid state. The hypothesis offers an explanation for the higher male variance in many aspects of cognitive performance (3). Genes on the X-chromosome not only influence general intelligence, but also have relatively specific effects on social–cognition and emotional regulation. Jamain et al. (4) described mutations in two X-linked genes encoding neuroligins NLGN3 and NLGN4 in siblings with autism spectrum disorders. One of these (NLGN4) was located at Xp22.3, a region where previously de novo deletions had been observed in autistic females (5). Subsequently, another family has been identified with similar phenotypic associations (6). We, therefore, have a potential explanation for the male preponderance of mental retardation in general, and for isolated heritable cases of autism in males, in particular. But, we are still some way from understanding the wider male predisposition to a range of neurodevelopmental disorders including reading disabilities (7), Asperger syndrome, which may be 10 times as common in males as in females (8) and attention deficit hyperactivity disorder (9). This review shall consider the accumulating evidence that there are several genetic and epigenetic mechanisms that could influence the role of X-linked genes in sexual dimorphism, not only in humans but also in mice, and thus potentially in other mammalian species too.
Mechanisms of sexual dimorphism involving X-linked genes
Genes on the X- and Y-chromosomes are of particular importance in the development of differences between the sexes, a fact that might at first sight appear self evident, because the mechanism for mammalian sex-specific differentiation involves the Y-linked gene SRY, but nature is not so transparent (10). The Y-chromosome does indeed contain a substantial proportion of genes that are involved in spermatogenesis (11,12). We might reasonably suppose these are on the Y-chromosome because this is evolution's way of ensuring they are expressed only in males. Surprisingly, many genes involved in spermatogenesis in mice are X-linked (13) and are expressed (exclusively) in males. How has this extraordinary situation evolved? Hurst (10) proposes that an X-linked locus is at least three times more likely to be involved in sexual development than is a locus on an autosomal chromosome, especially if that locus is advantageous to males. Accordingly, the X-chromosome could function as a filter for sexually antagonistic alleles. As the (male-advantageous) allele frequency increases on the X-chromosome, the proportion of females who are homozygous for that allele (which is disadvantageous to them) will also increase. Accordingly, deleterious gene-function will become suppressed in females. Logically, we should not be surprised to find a male-biased expression of X-linked genes in clearly sexually dimorphic processes such as spermatogenesis. The same mechanism may apply to specific higher cognitive functions, if they are associated with some male advantage in adaptation (1). Similarly, if there are mutations in such specialized genes associated with impaired function, these will be manifested more commonly in males than in females. Skewed patterns of X-inactivation may arise, which will influence the expression of recessive X-linked disease mutations in females. Skewing could also influence expression patterns of common allelic variants in genes that are subject to X-inactivation. Although skewing from the expected 50/50 ratio may occur simply by chance, extremely skewed inactivation patterns can result from mutations of the X-inactivation centre, or from large deletions of part of the X-chromosome. There is some limited and controversial evidence to suggest that skewing of X-inactivation normally becomes greater with advancing age, but the implications of that observation (if true) are unknown.
SEX-SPECIFIC X-LINKED GENES AND NEURAL DEVELOPMENT
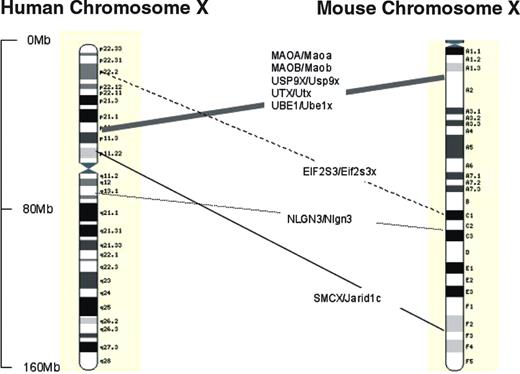
There is increasing evidence that some X-linked genes are expressed differently, depending on whether they are in male or female brains. The potential impact of Y-linked genes on sexual dimorphism is limited, because few different proteins are encoded by the Y-chromosome (12,14). Xu et al. (15) found that in mice, six X-linked homologues of Y-linked genes (Usp9x, Ube1x, Smcx, Eif2s3x, Utx and Dbx) were expressed in the brain at significantly higher levels in adulthood in females than in males, irrespective of their X-inactivation status. The X- and Y-homologues of three genes in particular, Usp9x/y, Ube1x/y and Eif2s3x/y, appeared to have acquired different functions and expression patterns in males and females. All three have human homologues, but their role in human neural development is presently unclear, although worthy of investigation (Fig. 1).
HUMAN GENES THAT ESCAPE X-INACTIVATION OUTSIDE THE PSEUDOAUTOSOMAL REGION
Eutherians (placental mammals) have very small regions of identical X–Y homology that remain capable of meiotic recombination. These regions contain relatively few genes (about 12) and are known as pseudoautosomal (PAR). Both X and Y copies are expressed in normal males and females (16), with presumed dosage equivalence. Most genes elsewhere on the randomly inactivated X-chromosome in females are silenced, but not all (17); the proportion escaping inactivation could be as many as 20% of the total (18). This is baffling at first sight, because X-inactivation must have evolved for the purpose of ensuring dosage equality in sex chromosome expressed genes between males and females of the species. Proportionately, the largest number of such ‘escapee’ genes lie on the short arm (Xp), which evolved relatively recently, the most distal region 30–50 million years ago, whereas eutheria diverged from metatheria (marsupials) 130 million years ago. Because the escapees are interspersed among genes that are subject to X-inactivation, they must be protected in some way. We do not currently know why or how this occurs although there are recent intriguing leads (19). Outside the PAR, there are only two Y-transposed genes with exact homologues on Xp (they have 99% identity to the X-linked copy) and a further 16 ‘degenerate’ genes, which are similar to the X-linked copy but they have different functions (12). Non-inactivated genes on the X-chromosome that lack a Y-homologue are potential candidates for sexual dimorphism (16,20). It should be possible to learn more about their functions in humans by studying females who have but a single X-chromosome and who would, therefore, be haploinsufficient for their products.
HUMAN X-MONOSOMY
In humans, partial or complete loss of one of the sex chromosomes, either the second X-chromosome or the Y-chromosome, results in X-monosomy (21). The fact that the condition, known clinically as Turner syndrome, is associated with a phenotype results from two main influences. First, there is haploinsufficiency for genes that are normally expressed from both X-chromosomes in females. They fall into two classes: genes in the pseudoautosomal regions (PAR1 and PAR2) and those outside the PAR which escape X-inactivation. Secondly, because non-inactivated genes contribute to the development and maintenance of ovarian tissues (22), there is early degeneration of the ovaries and consequent estrogen insufficiency. The condition is associated with short stature, which is due largely to haploinsufficiency for the SHOX gene, expressed from PAR1 (23). Other features include a high arched palate, neck webbing, broad chest, as well as characteristic cardiac and renal anomalies, but the genetic basis for such anomalies is not known. Textbook descriptions of Turner syndrome often exaggerate the severity of the associated physical anomalies because, until recently, most cases were identified in middle childhood and later-diagnosed cases tend to have more severe phenotypes (24). Occasionally, milder cases are not detected until adulthood—but these are likely to be mosaics rather than purely X-monosomic—about one-half of phenotypic Turner syndrome patients have detectable mosaicism for a second cell line. This additional cell line may contain a normal 46,XX karyotype (in which case the phenotypic features of the syndrome are ameliorated), some structural anomaly of the X-chromosome or, rarely, a partial Y-chromosome (lacking critical elements essential for the development of the male phenotype). In terms of cognitive development, girls with Turner syndrome have normal verbal intelligence, but they are deficient in terms of visuospatial abilities [such as the ability to complete a jigsaw puzzle (25)]. They also usually have difficulties in arithmetical abilities and may lack even a basic concept of number (26), implying dosage-sensitive X-linked genes are also involved in numerical cognitive skills and spatial intelligence.
SOCIAL–COGNITIVE DEVELOPMENT AND EMOTIONAL REACTIVITY IN X-MONOSOMIC FEMALES
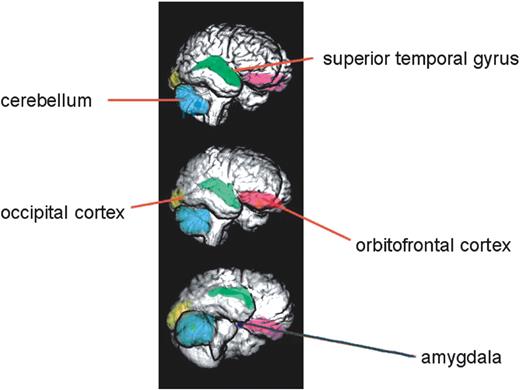
Impairments in social skills and affective discrimination affect the majority, who possess limited numbers of friends and who experience social isolation and a poor self-concept (27,28). The condition is associated with a substantially increased risk of autism (at least 200 times) (29). Focussed studies have demonstrated profound face and emotion recognition deficits in a minority (30), as well as difficulties in the interpretation of direction of other's eye gaze and line of sight (31). The nature and severity of these social–cognitive deficits points to an anomaly in the functioning of neural centres which, for many, is as severe as that reported in cases of bilateral amygdalectomy (32). Accordingly, there has been interest in the possibility that haploinsufficiency for one or more X-linked genes has a specific impact on development of the amygdala and its connections with cortical centres involved in social–cognition processing, the ‘social brain’ (33) (Fig. 2).
No genes that contribute to the cognitive or behavioural disorders of Turner syndrome have yet been identified. Recent research has, however, led to the delineation of a critical region on proximal Xp where a cluster of genes escapes X-inactivation (34), in which one or more candidate genes appear to be located. In a neuroimaging study of 45,X females, Good et al. (35) showed that the amygdala was structurally abnormal (enlarged). There were also increases in grey matter volume in the orbitofrontal cortex bilaterally, close to a region that is implicated in emotional learning. Intriguingly, the increase in amygdala size was even greater than the relative difference normally found between males and females (36,37). This implied that sexually dimorphic processes could be involved. Patients were selected who had variably sized deletions of the short arm of the X-chromosome, some of whom had the brain structural and functional deficits of X-monosomy. Mapping deletion size against phenotype, we identified a genetic locus 4.96 Mb in size at Xp11.3 that contained at least one dosage-sensitive X-linked gene influencing amygdala structure and function (35). Within this region lie a number of plausible candidate genes (Fig. 1). These include Usp9x, which escapes X-inactivation in humans as well as in mice (15), and the monoamine oxidase genes (MAOA and MAOB), which play an important role in psychiatric adjustment (38,39). MAOB enzymatic activity can be measured in platelets. It is sexually dimorphic, levels being ∼30% lower in males than in females. We found expression was even lower in 45,X females than in normal males (35) indicating the MAOB gene may escape X-inactivation and thus be haploinsufficient in both males and X-monosomic females.
IMPRINTING AND X-LINKED GENES IN TURNER SYNDROME
Because males invariably inherit their single X-chromosome from their mother, X-linked imprinted genes could theoretically have sexually dimorphic expression. This may arise because expression is exclusively from the paternally inherited X-chromosome (and thus only in females). Alternatively, expression could be exclusively from the maternally inherited X-chromosome and would be sexually dimorphic if the gene concerned was subject to X-inactivation. Skuse et al. (40) proposed, from a study contrasting X-monosomic females whose single X was either maternal or paternal in origin, that a paternally expressed allele was associated with enhanced social–cognitive abilities in normal females relative to males. X-linked imprinting could also protect females from deleterious allelic variants of autosomal genes that influence the functions of the social brain (41). Sexual dimorphism in the processing social perceptions and emotional responsiveness (42,43), involves amygdala-related neural circuitry (44). Although there is no simple correlation between individual social–cognitive variables and the parental origin of the single X-chromosome in X-monosomy, more complex relationships between such variables exist, which indicate the role of X-linked imprinting in social adjustment is rather more subtle than was at first suspected.
X-LINKED IMPRINTING AND BRAIN STRUCTURE
Kesler et al. (45) examined amygdala and hippocampal morphology in X-monosomic Turner females and looked specifically for differences in these brain structures according to the parental origin of the single X (Fig. 2). Previous work had demonstrated X-linked imprinting effects on the volumes of the superior temporal gyrus (46), as well as on occipital white matter and cerebellar grey matter (47). Good et al. (35) did not find an imprinting effect upon amygdala or frontal lobe structures. The Kesler et al. (45) sample was substantially larger, and was analyzed by a different methodology [manual delineation compared with voxel-based morphometry, (VBM)], but the results were similar. Despite replicating the Good et al. (35) findings of enlarged amygdala grey matter volumes, no impact of X-linked imprinting could be found upon structure. Recently, Cutter et al. (48) employed magnetic resonance imaging and proton magnetic resonance spectroscopy to investigate brain anatomy and metabolism in X-monosomy. Using both a hand-traced region of interest approach and VBM, 45,Xm women were shown to possess a significantly larger adjusted right hippocampal volume than 45,Xp subjects (personal communication), possibly explaining a prior finding that 45,Xp females have poorer visual memory than 45,Xm females, despite their better social adjustment (49). 45,Xm females had significantly smaller volume of caudate nucleus and thalamus than those with a single paternal X-chromosome. Dysfunction of the caudate nucleus could lead to abnormal executive function with impaired working memory, planning ability, set-shifting and social cooperation (50). Maternally expressed X-linked genes might, therefore, influence hippocampal development, and paternally expressed genes influence the normal development of the caudate nucleus and thalamus in females.
MOUSE MODELS OF X-MONOSOMY
Mice have proportionately far fewer genes that escape X-inactivation than do humans (19,51). It used to be thought that the X-monosomic mouse was not a good model for Turner syndrome (human X-monosomy) because they are fertile and do not have gross phenotypic anomalies in terms of growth or cognitive abilities. On the other hand, there are subtle differences in their behaviour, indicating that dosage-sensitive X-linked genes do influence cognitive and emotional processing in ways that are reminiscent of X-monosomic human females. 39,X mice can be generated by the fertilization of a normal gamete by a sex chromosome null gamete, and therefore are free from the problem of mosaicism, which might potentially influence the correct interpretation of X-monosomic data in humans (52). Isles et al. (53) reported that 39,X mice showed greater fear reactivity than 40,XX mice; they spent less time on the open arm of the elevated plus maze, a standard method for measuring anxiety in mouse models. The behaviour was not influenced by the stage of the oestrus cycle, locomotor activity, response to novelty or the parental origin of the single X-chromosome. Expression of the PAR gene Sts in the brains of 40,XX and 39,X mice was of particular interest, because reduced Sts expression could be antagonistic to GABAA receptors and hence theoretically evoke anxiogenesis. This hypothesis was not supported: in a partial X-deletion mouse model where Sts expression and expression levels of associated GABAA subunits were at least normal, increased fear reactivity persisted and appeared to be related to haploinsufficiency for a different (as yet unidentified) X-linked gene.
X-LINKED IMPRINTING IN X-MONOSOMIC MICE
Skuse et al. (40) presented evidence to indicate that there were differences in behavioural inhibition between X-monosomic females with respect to the parental origin of their single X-chromosome. 45,Xm subjects were less competent than either 45,Xp or 46,XX females at a simple task which required the inhibition of a prepotent response (54). Males were also less competent at the task than normal females. Davies et al. (55) studied samples of 39,X mice whose single X-chromosome was either of maternal or of paternal origin and looked for evidence of a parent-of-origin effect upon equivalent cognitive abilities. The Y-maze, a visual, non-spatial, serial reversal-learning paradigm was used, in which mice were trained to go down one of two goal arms to collect a foodstuff reinforcer. The arm containing the food might be either light or dark. After 85% correct responding over 3 days, the contingencies were reversed and errors recorded in terms of perseverative behaviour (going up the same unrewarding arm persistently) and the formation of new reinforcer-stimulus associations (correct responding after the switch). 39,Xm0 mice showed deficits in reversal learning, but there were no significant differences in performance between the 39,Xp0 mice and the 40,XX mice. The same authors have compared neural gene expression in the two sets of monosomic mice by microarray analysis to reveal a potentially maternally expressed X-linked imprinted candidate gene, the characterisation of which is ongoing (56). Studies are currently underway to discover whether functionally similar genes on the human X-chromosome are also subject to parent-of-origin specific expression in a study of X-monosomic females.
CONCLUSIONS AND FUTURE DIRECTIONS
There has, in recent years, been a substantial number of disorders identified which are associated with non-syndromic or ‘pure’ mental retardation, associated with a rapidly increasing collection of cloned ‘X-linked mental retardation’ (XLMR) genes (59). For reasons that are not yet understood, there is an excess proportion of genes on the X-chromosome that are associated with the development of intelligence, with no obvious links to other significant biological functions. Mutations in autosomal genes that are associated with mental retardation often accompany somatic anomalies or overt disruption to structural brain development; they are ‘syndromic’ in character, unlike up to two-thirds of mutations in XLMR genes (60). Recent work has suggested that, in the critical Xp11 region that harbours a large number of such XMLR genes (59), there may be others specialized for abilities such as social intelligence too. Perhaps subtle polymorphic variations in genes that, when non-functional, lead to serious learning difficulties can have relatively specific modulating influences on intellectual or social abilities (35). A key implication of these findings is that male and female brains may differ not only because of their contrasting genetic constitutions, but also because of their sex-steroid environments, and that differences in cognitive and social abilities between the sexes could be directly linked to the influence of X-chromosome genes. Recently, we have learned that there is remarkable traffic, in terms of retrotransposition of genes in both directions, between the X-chromosome and the autosomes; sexual antagonism and sex-biased gene expression may be explicable in terms of this remarkable phenomenon (61). A particularly exciting possibility is that genes which are involved in relatively subtle influences upon behaviour in rodents (54) have evolved to modulate human social responses too or were acquiring new cognition-related functions in primates (62).
ACKNOWLEDGEMENTS
Work by D.H.S. has been funded by the Wellcome Trust, the National Alliance for Autism Research, the Child Growth Foundation and the Nancy Lurie Marks Family Foundation.
Figure 1. Relative positions of genes discussed, where known orthologies exist on mouse and human X-chromosomes. Physical distances are shown as marked. Approximate mouse and human map positions from: Mouse Chromosome X Linkage Map with Human Orthologies, Mouse Genome Database (www.informatics.jax.org), database update 18/01/2005 MGI3.1. Confirmatory positions from Ensembl (www.ensembl.org) release 27.35a.1 updated 14/01/2004 (human) and 27.33c.1 (mouse). Images adapted from Ensembl.
Figure 2. Renderings of a normal human brain, reconstructed from serial magnetic resonance images, showing some of the neuroanatomical structures discussed in this review that are influenced by X-linked genes. Other structures that are discussed, but which are not visible in these images because they lie more deeply in the brain, include the hippocampus, thalamus and caudate nuclei. The amygdala is represented bilaterally and lies deeply in the mesial temporal lobe; it is closely associated anatomically with the hippocampus, the caudate nuclei and the thalamus. Many of these structures comprise elements of the so-called social brain (56,57). Figure originally provided by Deema Fattal and Hanna Damasio, Human Neuroanatomy and Neuroimaging Laboratory, Department of Neurology, University of Iowa, and adapted from Adolphs (56) and reproduced with permission.
References
Graves J.A., Gecz, J. and Hameister, H. (
Zechner, U., Wilda, M., Kehrer-Sawatzki, H., Vogel, W., Fundele, R. and Hameister, H. (
Hedges, L.V. and Nowell, A. (
Jamain, S., Quach, H., Betancur, C., Rastam, M., Colineaux, C., Gillberg, I.C., Soderstrom, H., Giros, B., Leboyer, M., Gillberg, C. et al. (
Thomas, N.S., Sharp, A.J., Browne, C.E., Skuse, D., Hardie, C. and Dennis, N.R. (
Laumonnier, F., Bonnet-Brilhault, F., Gomot, M., Blanc, R., David, A., Moizard, M.P., Raynaud, M., Ronce, N., Lemonnier, E., Calvas, P. et al. (
Rutter, M., Caspi, A., Fergusson, D., Horwood, L.J., Goodman, R., Maughan, B., Moffitt, T.E., Meltzer, H. and Carroll, J. (
Hermens, D.F., Williams, L.M., Lazzaro, I., Whitmont, S., Melkonian, D. and Gordon, E. (
Hawley, R.S. (
Skaletsky, H., Kuroda-Kawaguchi, T., Minx, P.J., Cordum, H.S., Hillier, L., Brown, L.G., Repping, S., Pyntikova, T., Ali, J., Bieri, T. et al. (
Wang, P.J., McCarrey, J.R., Yang, F. and Page, D.C. (
Arnold, A.P. and Burgoyne, P.S. (
Xu, J., Burgoyne, P.S. and Arnold, A.P. (
Craig, I.W., Mill, J., Craig, G.M., Loat, C. and Schalkwyk, L.C. (
Brown, C.J., Greally, J.M. (
Carrel, L., Cottle, A.A., Goglin, K.C. and Willard, H.F. (
Fillipova, G.N., Cheng, M.K., Moore, J.M., Truong, J.P., Hu, Y.J., Nguyen, D.K., Tsuchiya, K.D.and Disteche, C.M. (
Craig, I.W., Harper, E. and Loat, C.S. (
James, R.S., Coppin, B., Dalton, P., Dennis, N.R., Mitchell, C., Sharp, A.J., Skuse, D.H., Thomas, N.S. and Jacobs, P.A. (
Rao, E., Weiss, B., Fukami, M., Rump, A., Niesler, B., Mertz, A., Muroya, K., Binder, G., Kirsch, S., Winkelmann, M. et al. (
Gunther, D.F., Eugster, E., Zagar, A.J., Bryant, C.G., Davenport, M.L. and Quigley, C.A. (
Temple, C.M. and Carney, R.A. (
Molko, N., Cachia, A., Riviere, D., Mangin, J.F., Bruandet, M., LeBihan, D., Cohen, L. and Dehaene, S. (
McCauley, E., Feuillan, P., Kushner, H. and Ross, J. (
Ross, J., Zinn, A. and McCauley, E. (
Creswell, C. and Skuse, D. (
Lawrence, K., Kuntsi, J., Coleman, M., Campbell, R. and Skuse, D. (
Lawrence, K., Campbell, R., Swettenham, J., Terstegge, J., Akers, R., Coleman, M. and Skuse, D. (
Adolphs, R. (
Skuse, D.H., Morris, J. and Lawrence, K. (
Brown, C.J. and Greally, J.M. (
Good, C.D., Lawrence, K., Thomas, N.S., Price, C.J., Ashburner, J., Friston, K.J., Frackowiak, S.J., Oreland L. and Skuse, D.H. (
Good, C.D., Johnsrude, I., Ashburner, J., Henson, R.N., Friston, K.J. and Frackowiak, R.S. (
Goldstein, J.M., Seidman, L.J., Horton, N.J., Makris, N., Kennedy, D.N., Caviness, V.S., Jr, Faraone, S.V. and Tsuang, M.T. (
Oreland, L., Hallman, J. and Damberg, M. (
Caspi, A., McClay, J., Moffitt, T.E., Mill, J., Martin, J., Craig, I.W., Taylor, A. and Poulton, R. (
Skuse, D.H., James, R.S., Bishop, D.V., Coppin, B., Dalton, P., Aamodt-Leeper, G., Bacarese-Hamilton, M., Creswell, C., McGurk, R. and Jacobs, P.A. (
Skuse, D.H. (
Canli, T., Desmond, J.E., Zhao, Z. and Gabrieli, J.D. (
Cahill, L., Gorski, L., Belcher, A. and Huynh, Q. (
Campbell, R., Elgar, K., Kuntsi, J., Akers, R., Terstegge, J., Coleman, M. and Skuse, D. (
Kesler, S.R., Garrett, A., Bender, B., Yankowitz, J., Zeng, S.M. and Reiss, A.L. (
Kesler, S.R., Blasey, C.M., Brown, W.E., Yankowitz, J., Zeng, S.M., Bender, B.G. and Reiss, A.L. (
Brown, W.E., Kesler, S.R., Eliez, S., Warsofsky, I.S., Haberecht, M., Patwardhan, A., Ross, J.L., Neely, E.K., Zeng, S.M., Yankowitz, J. et al. (
Cutter, W.J., Robertson, D.M., Daly, E., Ng, V., Conway, G. and Murphy, D.G. (
Bishop, D.V., Canning, E., Elgar, K., Morris, E., Jacobs, P.A. and Skuse, D.H. (
Rilling, J., Gutman, D., Zeh, T., Pagnoni, G., Berns, G. and Kilts, C. (
Tsuchiya, K.D. and Willard, H.F. (
Isles, A.R., Davies, W., Burrmann, D., Burgoyne, P.S. and Wilkinson, L.S. (
Manly, T., Anderson, V., Nimmo-Smith, I., Turner, A., Watson, P. and Robertson, I.H. (
Davies, W. (
Davies, W., Isles, A., Smith, R., Burgoyne, P. and Wilkinson, L. (
Calder, A.J., Lawrence, A.D. and Young, A.W. (
Fishburn, J., Turner, G., Daniel, A. and Brookwell, R. (
Khil, P.P., Oliver, B. and Camerini-Otero, R.D. (
Shoichet, S.A., Hoffmann, K., Menzel, C., Trautmann, U., Moser, B., Hoeltzenbein, M., Echenne, B., Partington, M., van Bokhoven, H., Moraine, C. et al. (