-
PDF
- Split View
-
Views
-
Cite
Cite
Ichiko Nishijima, Takanori Yamagata, Corinne M. Spencer, Edwin J. Weeber, Olga Alekseyenko, J. David Sweatt, Mariko Y. Momoi, Masayuki Ito, Dawna L. Armstrong, David L. Nelson, Richard Paylor, Allan Bradley, Secretin receptor-deficient mice exhibit impaired synaptic plasticity and social behavior, Human Molecular Genetics, Volume 15, Issue 21, 1 November 2006, Pages 3241–3250, https://doi.org/10.1093/hmg/ddl402
Close - Share Icon Share
Abstract
Secretin is a peptide hormone released from the duodenum to stimulate the secretion of digestive juice by the pancreas. Secretin also functions as a neuropeptide hormone in the brain, and exogenous administration has been reported to alleviate symptoms in some patients with autism. We have generated secretin receptor-deficient mice to explore the relationship between secretin signaling in the brain and behavioral phenotypes. Secretin receptor-deficient mice are overtly normal and fertile; however, synaptic plasticity in the hippocampus is impaired and there are slightly fewer dendritic spines in the CA1 hippocampal pyramidal cells. Furthermore, secretin receptor-deficient mice show abnormal social and cognitive behaviors. These findings suggest that the secretin receptor system has an important role in the central nervous system relating to social behavior.
INTRODUCTION
Secretin is a 27 amino acid peptide belonging to the secretin-glucagon peptide hormone family (1). Secretin was originally isolated from the duodenum to secrete pancreatic juice, a mixture of products including amylase, lipase, other proteases and bicarbonate (1,2). It was recently reported that secretin was also produced in the cerebellum (3), hippocampus and the area postrema of the medulla (4). The receptor for secretin is a G-protein-coupled receptor that is expressed in the pancreas (5), stomach (6), kidney (7) and brain [cerebellum, hippocampus and central amygdala (4,8)]. Secretin receptor is a member of the type II G-protein-coupled receptor family that also includes the VIP receptors (VPAC1 and VPAC2), the PACAP receptor (PAC1), the glucagon receptor and the glucagon-like peptide receptors (GLP1R and GLP2R) (9). Secretin can bind these related receptors with lower affinity, and the corresponding peptide hormones in this family can bind the secretin receptor (10–12). The secretin receptor, VIP receptors and the PACAP receptor have similar effects in that they all elevate intracellular cAMP by coupling to adenylate cyclase through a Gs heteromeric G-protein (13–15). In addition, they affect phospholipase C activation, intercellular Ca++ release and mitogen-activated protein (MAP) kinase activation through Gq- (16) and Gi-proteins (17). However, the expression pattern for each of these receptors is slightly different (18,19). VIP and PACAP and their receptors have been reported to be involved in several central nervous system (CNS) functions such as emotion and recognition (20). Both secretin and its receptor have been observed in the CNS (4,21,22), but the function of this ligand-receptor pair in this context has not been established.
In 1998, Horvath et al. (23) described improved behavioral and language skills in autistic children who received porcine secretin to test their gastrointestinal function. Subsequent controlled studies have either failed to confirm the results of the Horvath study or have shown some behavioral improvements among a subset of children with autism (24,25). Secretin has also been described as being potentially therapeutic for other behavioral disorders such as schizophrenia (26,27).
We have generated secretin receptor-deficient mice to examine the role of this gene in the CNS and to explore whether the secretin-receptor system plays a role in neurobehavioral phenotypes. We found that the secretin receptor is important for hippocampal synaptic plasticity and social and cognitive behavior.
RESULTS
Generation of secretin receptor-deficient mice
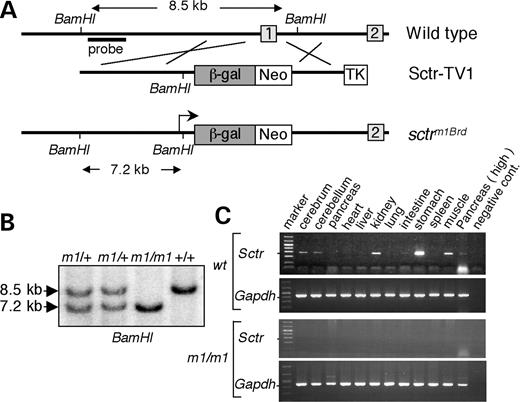
To analyze the function of the secretin receptor in vivo, we generated secretin receptor-deficient mice using embryonic stem cell technology. A targeting vector was constructed which was designed to replace exon 1 of the secretin receptor with the lacZ reporter and a PGKneobpA selection marker (Fig. 1A). ES cells with a targeted allele were obtained at a rate of 10% following electroporation of the targeting vector in the AB2.2 ES cell line. This allele, termed sctrm1Brd (m1), was established in mice using standard procedures. Intercross of m1/+ mice generated m1/m1 homozygous mutants at the expected 25% frequency (Fig. 1B). Secretin receptor-deficient mice were fully viable and fertile and had normal body weight. RT–PCR analysis of tissues which normally express secretin receptor mRNA (cerebrum, cerebellum, kidney, muscle, stomach and pancreas) revealed no expression of secretin receptor in m1/m1 mice, confirming that the m1 allele was null (Fig. 1C). We examined the kidney, stomach and pancreas of the mutant mice histologically. All pancreata had multiple variably sized islets. Acinar cells had typical zymogen granules and the ducts contained secretory material. No cell death, degeneration or inflammation was seen. The stomachs had a normal fundic epithelium, with normal-appearing chief, parietal and foveolar cells. The kidneys were unexceptional in appearance (data not shown).
Generation of secretin receptor-deficient mice. (A) The targeting vector (Sctr-TV1) was designed to replace exon 1 of the secretin receptor with the lacZ reporter and a PGKneobpA selection marker. (B) Southern blot analysis distinguishes the secm1Brd and wild-type alleles. The BamHI restriction fragment length of the wild-type allele is 8.5 kb and m1 allele is 7.2 kb. (C) RT–PCR analysis of expression of secretin receptor in different tissues from secretin receptor-deficient mice and their wild-type littermates.
Neuropathological examination and LacZ reporter expression
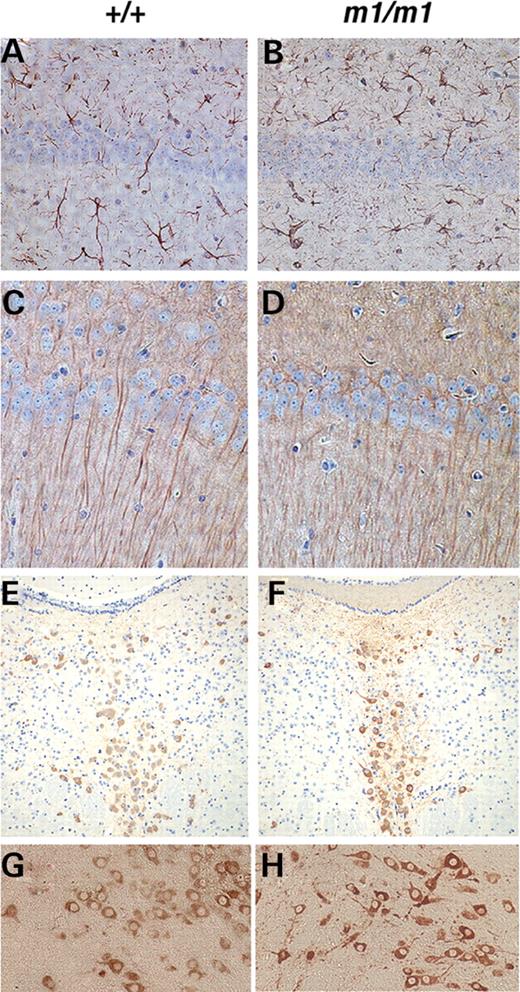
We examined sections of the brain in secretin receptor-deficient adult mice for neuroanatomical changes with standard hematoxylin–eosin stains and immuno-histochemistry using antibodies to neurofilament, glial fibrillary acidic protein (GFAP), MAP2, tryptophan hydroxylase and tyrosine hydroxylase. Hematoxylin–eosin stains revealed no obvious malformation or degeneration in secretin receptor-deficient brain sections. Changes in structural proteins of the pyramidal neurons were examined using antibodies to MAP2 and alterations in neurotransmitters were analyzed using antibodies to tyrosine hydroxylase and tryptophan hydroxylase. The staining patterns of these markers were indistinguishable between the secretin receptor-deficient mice and their wild-type littermates (Fig. 2C–H). There was no evidence of a glial reaction as determined using antibodies to GFAP (Fig. 2A and B). These results suggest that, using these techniques, the neuroanatomy in the mutants and wild-type littermates is similar. However, analysis of the dendritic spines in the CA1 region of the hippocampus does reveal an abnormality in the secretin receptor-deficient mice.
Neuronal and glial marker analysis in adult brain of secretin receptor-deficient mice. Similar expression patterns are observed in both secretin receptor-deficient and wild-type littermates detected by immuno-histochemistry using antibodies to (A and B) GFAP in the hippocampal region; (C and D) MAP2 in the hippocampal region; (E and F) tryptophan hydroxylase in the raphe nucleus; (G and H) tyrosine hydroxylase in the substantia nigra. (A, C, E and G) Wild-type littermates and (B, D, F and H) secretin receptor-deficient mice.
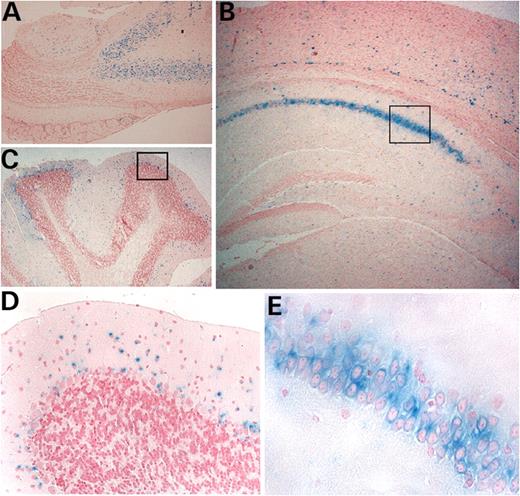
To analyze the expression of the secretin receptor, we stained the brains of secretin receptor m1/+ and m1/m1 mice with X-Gal. We found strong expression of the secretin receptor in the hippocampal CA1 region, the lower layer of cerebral cortex, the anterior olfactory nuclei, the anterior ventrolateral thalamus, the lateral region of hypothalamus, substantia nigra, tegmental area and central nucleus of the inferior colliculus, the ventral supramamillary nucleus and the cerebellum (Fig. 3).
Secretin receptor expression in adult brain revealed by staining of the lacZ reporter. The expression pattern was not different between secretin receptor m1/+ and m1/m1 mice. Expressions in (A) anterior olfactory neuron, (B) hippocampal CA1 region and (C) cerebellum. (D) Enlargement of box area in (C). (E) Enlargement of box area in (B).
Impaired synaptic transmission and long-term potentiation in secretin receptor mutant mice
To better understand the role of secretin in the hippocampus, the well-defined Schaffer collateral synapses were examined for altered synaptic function. Synaptic transmission was determined from population excitatory postsynaptic potentials (pEPSPs) of field recordings from the hippocampal CA1 synapses. These were evaluated by determining the amplitude of the evoked fiber volley versus the slope of the field EPSP at increasing stimulus intensities (Fig. 4A). Loss of the secretin receptor appears to have a deleterious effect on overall synaptic transmission at Schaffer collateral synapses. Figure 4A shows a significant difference when an F-test is performed on the best fit curves (F[2,16]=4.095, P=0.0366; m1/m1, n=21; wild-type, n=11). Interestingly, the reduction in synaptic transmission for m1/m1 mice was due to a decrease in the slope of the pEPSP at increasing stimulus intensities and not due to a change in the fiber volley amplitude (data not shown). Since the amplitude of the fiber volley is a measurement of presynaptic firing and the slope of the pEPSP is a measurement of the postsynaptic response to stimulation, these results suggest that the reduced synaptic transmission is a result of miscommunication across the Schaffer collateral synapse, which is not due to a deficit in presynaptic terminal firing.
Impaired synaptic transmission and LTP of hippocampal CA1 slices. (A) Synaptic transmission was determined from pEPSPs of field recordings from the hippocampal CA1 synapses. Loss of the secretin receptor appears to have a deleterious effect on overall synaptic transmission (F[2,16]=4.095, P=0.0366, m1/m1, n=21; wild-type, n=11). (B) There were no significant changes in the short-term synaptic plasticity by testing the paired-pulse facilitation (PPF). (C) Deficiency of hippocampal LTP induction and maintenance phase in secretin receptor m1/m1 was observed (two-way ANOVA: genotype: F[1,481]=83.97, P<0.0001; time: F[25,481]=1.90, P<0.0001, m1/m1, n=13; +/+, n=11).
In light of the deficit in synaptic transmission, we sought to determine whether there was a change in the short-term facilitation in the CA1 area by examining paired-pulse facilitation (PPF). PPF is the facilitation of neurotransmitter release likely due to residual calcium present in the presynaptic terminal following depolarization (28) and is likely to be involved in some forms of learning and memory (29). Since PPF varies with the stimulus intensity and the size of the pEPSP of the first response, the stimulus intensity was adjusted so that the slopes of the pEPSP from the first stimulus for both secretin receptor-deficient and wild-type mice were the same (data not shown). Normalizing the slopes of the first response was particularly important in light of the difference in input/output in secretin receptor-deficient mice. The percent of PPF was determined at interpulse intervals of 20, 50, 100, 200 or 300 ms. m1/m1 mutants (n=20) showed no significant deficits in PPF compared with +/+ mice (n=12) at any of the interpulse intervals tested (Fig. 4B). This suggests that the mechanisms controlling PPF in secretin receptor-deficient mice is intact and supports the hypothesis that presynaptic function is intact in secretin receptor-deficient mice.
Hippocampal long-term potentiation (LTP) is an use-dependent increase in synaptic efficacy believed to be similar to the processes underlying the long-term memory formation in mammals. Given that the absence of the secretin receptor appears to be responsible for the deficits in synaptic transmission, we sought to examine the possibility that this would also affect long-lasting synaptic plasticity. Hippocampal LTP was induced by stimulation of the Schaffer collateral pathway in the CA1 area with a modest protocol consisting of two trains of tetani (2×1 s, 100 Hz, separated by 20 s.). We observed a significant reduction in overall LTP induction in secretin receptor-deficient mice immediately following high-frequency stimulation and throughout the induction and maintenance phases of LTP (two-way ANOVA: genotype: F[1,481]=83.97, P<0.0001; time: F[25,481]=1.90, P<0.0001, m1/m1, n=13; +/+, n=11) (Fig. 4C). These results demonstrate that secretin receptors are necessary for normal LTP induction in the CA1 area of the hippocampus.
Reduced dendritic spine number in secretin receptor-deficient mice
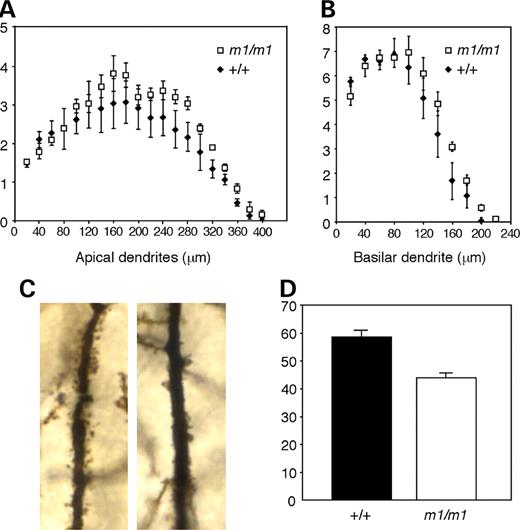
One possible cause of reduced synaptic transmission is an alteration in dendritic morphology or spine density. Therefore, we compared the structure of the dendritic tree and the dendritic spine density between secretin receptor-deficient mice and their wild-type littermates. Sholl analysis revealed that the average number of dendritic branches in CA1 pyramidal neurons in the mutant mice was similar to the number in the wild-type mice for both apical and basal dendrites (P-values >0.05) (Fig. 5A and B). However, there were significantly fewer spines on CA1 apical dendrites in secretin receptor mutant mice (Fig. 5C). The average dendritic spine number for the wild-type animals was 58.6 spines per 100 µm of dendrite and for the secretin receptor-deficient mice was 43.9 spines (unpaired t-test, n=30 per group, P<0.0001) (Fig. 5D). These results suggest that the reduced ‘postsynaptic’ synaptic transmission displayed by the mutant mice correlates with a reduction in spine density.
Reduced dendritic spine number in secretin receptor-deficient mice. The average number of apical (A)and basilar (B) dendritic branches of CA1 pyramidal neurons in mutant mice was similar to the number in wild-type mice. (C) Higher magnification images of apical dendrites in the CA1 pyramidal neurons revealed decreased spine density in the mutant (right) versus wild-type littermates (left). (D) The average number of dendritic spines on 100 µm of the first order apical dendritic branches in mutant mice was significantly reduced when compared with the average number of dendritic spines at similar sites in wild-type littermates (P<0.0001).
Reversal water maze behavior abnormalities in secretin receptor mutant mice
To examine behavioral consequences of reduced dendritic spine number and the defect in synaptic plasticity in the hippocampus, secretin receptor-deficient mice were tested in the Morris water maze, which is a hippocampal-dependent spatial memory test. In this hidden platform test, the mice learn the spatial relationships between objects in the room and the position of the platform they can use to escape from the water. Both the secretin receptor-deficient mice and the control mice showed significant reductions in latency (search time) over the eight blocks of training. There were no significant differences between the mutants and the wild-type mice (P-values >0.05) (Fig. 6A). However, in the reversal platform test, which tests the ability to learn the position of the hidden platform that has been moved to the opposite quadrant of its original location, secretin receptor-deficient mice did not show a decrease in latency over the four blocks of the test compared with the wild-type mice (two-way ANOVA: genotype: F[1,43]=8.656, P<0.005; time: F[3,129]=3.828, P<0.02) (Fig. 6B).
Reversal water maze behavior abnormalities in secretin receptor mutant mice. (A) Hidden platform test (latency). Both secretin receptor-deficient mice (m1/m1) and wild-type littermates (+/+) showed significant reductions in latency (search time) over the eight blocks of training. There was no significant difference between m1/m1 and +/+ (P>0.05). (B) Reversal hidden platform test (latency). Secretin receptor-deficient mice (m1/m1) did not show a decrease in latency over the four blocks of the test when compared with the wild-type mice (+/+) (two-way ANOVA: genotype: F[1,43]=8.656, P<0.005; time: F[3,129]=3.828, P<0.02).
Social behavior abnormalities in secretin receptor mutant mice
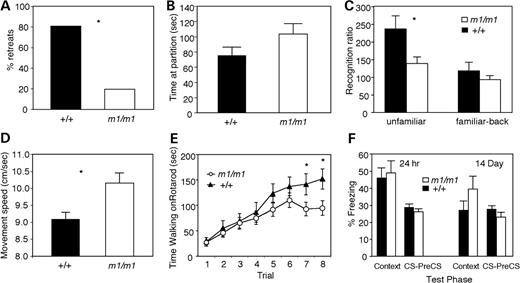
As an initial screen for normal social behavior, mice were tested in a tube test for social dominance (30,31). We have previously found this simple test to be useful in predicting impairments in social interaction (32,33). In this test, one mutant mouse and one wild-type mouse from different home cages were placed at opposite ends of a tube. A mouse was declared a ‘winner’ when the opposing mouse backed out first. We performed 39 matches with 13 mutant and 13 wild-type mice. Secretin receptor-deficient mice ‘won’ significantly more (29/36 or 81%) of their matches against wild-type littermates than expected by chance (χ2=13.4, P<0.001) (Fig. 7A). Thus, secretin receptor-deficient mice showed abnormal social behavior. To study this further, we evaluated their responses in the partition test for social interest and recognition. The advantage of utilizing the partition test for social behavior analysis comes from the ability to assess sociability without direct physical contact (34,35). The time spent near the partition during the baseline assessment with the original partner was not significantly different between secretin receptor-deficient and wild-type mice (P>0.05) (Fig. 7B). For the wild-type mice, the time spent at the partition when paired with an unfamiliar partner was 136% more than the time spent when paired with the original partner. In contrast, the secretin receptor-deficient mice only increased their time at the partition by 38% in the presence of an unfamiliar partner. Thus, the unfamiliar recognition ratio was significantly lower (P<0.03) in secretin receptor-deficient mice compared with wild-type mice (Fig. 7C). In contrast, when the initial familiar partner was returned into the cage, the recognition ratio was similar between the two genotypes (P>0.05). These findings indicate that secretin receptor-deficient mice have impaired social recognition behavior in the partition test.
Secretin receptor-deficient mice showed abnormal social behavior. (A) Tube test of social dominance. Secretin receptor-deficient mice won more matches in the tube test against wild-type littermates than expected by chance (P<0.001). (B) Partition test of social recognition. Time near the partition during the familiar partner baseline test was not significantly different between secretin receptor-deficient and wild-type mice. (C) Secretin receptor-deficient mice displayed a significantly lower recognition ratio compared with the wild-type mice during the test with unfamiliar partner (P<0.03). Data are expressed as the mean+/−SEM. (D) Locomotor activity of the secretin receptor-deficient mice. There was a significant difference in the movement speed when compared with wild-type littermates. (E) Secretin receptor-deficient mice performed as well as wild-type controls on the rotarod initially, but by the end of training, the secretin receptor-deficient mice were impaired (trial×genotype interaction, P=0.045; trial 1–6, P-values >0.05; trials 7–8, P-values <0.05). (F) Secretin receptor-deficient mice showed no significant difference in conditional fear during the test after a 24 h or 14-day delay.
Locomotor activity and motor skills of secretin receptor-deficient mice
Locomotor activity of secretin receptor-deficient mice was evaluated by open-field test. Secretin receptor-deficient mice moved significantly faster than wild-type mice in the open-field test (Fig. 7D), suggesting hyperactivity. Other open-field measurements such as distance traveled, time spent moving and rearing responses were not significantly different between m1/m1 and wild-type mice (P-values >0.05). Motor coordination and skill learning were assessed on the accelerating rotarod. Wild-type and m1/m1 mice performed similarly on the first day of testing; however, the secretin receptor-deficient mice performed significantly worse than wild-type mice during the last trials (Fig. 7E) on the second day of training (trial X genotype interaction, P=0.045; trials 1–6, P-values >0.05; trials 7–8, P-values <0.05).
Normal conditional fear in secretin receptor-deficient mice
Mice were tested for their ability to learn and remember an association between a training context and a footshock, which served as unconditioned stimulus, and between an auditory conditioned stimulus (CS) and the footshock. Freezing behavior was assessed during training and during the context and auditory CS tests 24 h and 2 weeks after training. Secretin receptor-deficient mice showed significantly more freezing than wild-type littermates during the first 2 min in the conditioning chamber prior to the first footshock (m1/m1 freezing, 4.5%; wild-type freezing, 0.0%; P=0.006). However, there were no differences (P-values >0.05) between the mutant mice and their wild-type littermates in the levels of freezing during the context tests or the auditory CS tests either 24 h or 2 weeks after training (Fig. 7F).
DISCUSSION
In this study, we generated a novel mouse strain that is deficient in the secretin receptor gene. Secretin receptor-deficient mice appeared normal in their home cages, and a histological analysis revealed no gross anatomical abnormalities in the brain. However, electrophysiological, dendritic morphological and behavioral analyses revealed that secretin receptor-deficient mice have abnormal brain functions.
We analyzed the neuronal function of secretin receptor-deficient mice using an electrophysiological approach. Hippocampal LTP is an use-dependent increase in synaptic efficacy believed to be similar to processes underlying the long-term memory formation in mammals. In secretin receptor-deficient mice, postsynaptic transmission was decreased. In addition, there was a significant reduction in LTP induction and LTP maintenance. These results suggest that secretin receptors are necessary for normal hippocampal function. Electrophysiological analysis of hippocampal slices from secretin deficient mice revealed a deficiency in synaptic transmission, which appears to result from miscommunication between the CA3 Schaffer collateral and CA1 pyramidal neurons. To study this morphologically, we measured dendritic branching patterns and dendritic spine numbers of the pyramidal neurons in CA1. The branching pattern of dendrites in the secretin receptor-deficient mice is same as in the wild-type mice; however, in the mutant mice, the dendritic spine numbers on the branches of the CA1 apical dendrites were significantly reduced. The dendritic spine is the primary place for connecting excitatory neuronal axons and for synaptic modification. Reduced dendritic spine numbers may induce reduced postsynaptic transmission, which may cause a significant reduction in LTP induction and LTP maintenance. Secretin receptor signaling regulates cAMP elevation through Gs-protein and activation of cAMP-regulated protein kinase (PKA) (13–15). Murphy and Segal (36) demonstrated that activation of cAMP responsive element binding protein (CREB) mediates generation of new dendritic spines. These results suggest that PKA-CREB pathways through secretin receptors may influence spine formation. Autism (37,38), Rett syndrome (39), most forms of mental retardation (40–42) and neurodegenerative disorders (43,44) show correlation with the reduction of dendrite complexity and the electrophysiological findings of miscommunication of the CA1 dendrite. Thus, it is possible that alterations in secretin signaling contribute to some features of neurobehavioral abnormalities by influencing the mechanisms of synapse formation.
The behavioral analysis demonstrates that secretin receptor-deficient mice exhibit altered social behaviors. The social interaction abnormalities were detected in two different assays. First, secretin receptor-deficient mice were less likely to retreat in the tube test. Individuals with Rett syndrome caused by mutations in MECP2 display autistic-like abnormal social interactions. Interestingly, Mecp2 mutant mice (32) display the same phenotype in the tube test as the secretin receptor-deficient mice. Tube-test matches took two to three times longer to resolve with both Mecp2 and secretin receptor-deficient mice than with other strains of mice, suggesting that these mutant mice are less likely to retreat from the tube (32,35). We hypothesize that rigid behavioral phenotypes in these mutant mice reduce the likelihood that they will back out of the tube. Since secretin receptor-deficient mice have normal anxiety-related responses in the open-field and light–dark tests (data not shown), it is unlikely that the abnormal tube-test response is due to stress or anxiety effects. Furthermore, in the partition test, which is a social response test, secretin receptor-deficient mice displayed normal exploration of the partition with a familiar partner, but decreased social recognition of an unfamiliar partner. These results suggest that secretin receptor-deficient mice have impaired social recognition behavior. PACAP receptor (PAC1) is a member of the type II G-protein-coupled receptor family including secretin receptor, and PAC1-deficient mice have an altered social behavior phenotype (45). It may be possible that downstream signals through common G proteins could result in the behavioral abnormalities.
In addition to social behavior abnormalities, secretin receptor-deficient mice exhibit altered cognitive behaviors. The performance of secretin receptor-deficient mice in the Morris water maze during the eight blocks of training was similar to that of wild-type littermates; however, there is a significant deficit in the reversal learning test. Reversal learning involves two processes: inhibiting the previously learned response and acquiring a new response. Autistic patients exhibit repetitive behaviors which they often refuse to change (46), and the reversal task of the Morris water maze test is believed to provide a good assessment of autistic repetitive behaviors in mice (47). The results of the reversal learning test indicate to measure that secretin receptor-deficient mice may have repetitive behaviors which consist of a failure to stop searching for the initial platform position, and consequently, they fail to develop new strategies for the new platform position. The relationship between secretin receptor downstream signals and behavioral output will be studied in the future.
We found expression of the secretin receptor in various areas of the brain, including the hippocampus, cerebral and the cerebellum. Although secretin receptor has been observed in rat hippocampus previously (4,21), we found the high level of expression of the secretin receptor specifically in the hippocampal CA1 region. Others have reported that secretin receptor mRNA is expressed in Purkinje cells and GABAergic interneurons in the cerebellum (8). These mice could provide utility for investigating the function of secretin in these other neuronal populations.
Autism is a neurodevelopmental disorder characterized by impaired social interaction and communication, with delayed or altered language development and restricted, repetitive and stereotypic behavior (48). Family and twin studies have suggested that genetic factors contribute to the development of autism (49). A significant proportion of individuals with single gene defects that result in mental retardation such as fragile X syndrome, tuberous sclerosis and Rett syndrome show autistic symptoms (50,51). Although a role of secretin in the etiology of autism has not been established, our secretin receptor-deficient mice have provided clear evidence that the secretin receptor plays an important role in the CNS function. Secretin receptor-deficient mice may provide an animal model to study the downstream signaling cascades that lead to neuronal and behavioral abnormalities and may potentially be useful tools for improved therapeutic intervention of autistic symptoms.
MATERIALS AND METHODS
Generation of secretin receptor-deficient mice
The mouse genomic DNA containing the first methionine codon of the secretin receptor gene was isolated from a 129S5 genomic DNA library, using a rat secretin receptor exon 1 cDNA probe. Isolated genomic DNA was assembled into a gene targeting vector, which included the E. coli beta-galactosidase (lacZ) and PGKneobpA markers. Appropriate gene targeting replaces the endogenous secretin receptor gene with lacZ. AB2.2 ES cells (129S7 strain) were cultured, electroporated and selected in G418 (350 µg/ml). Isolated clones were screened by Southern analysis as previously described (52). ES cells with the targeted allele were injected into C57BL/6J-Tyrc-Brd blastocysts according to standard protocols (53). Male chimeric mice were mated with female C57BL/6-Tyrc-Brd mice to establish the secretin receptor-deficient allele in the mouse germline. All mice were examined on a C57BL/6J×129S7 mixed genetic background.
RT–PCR for studying secretin receptor expression
Total RNA was isolated from brain (cerebral and cerebellum), pancreas, stomach, intestine (duodenum), lung, liver, skeletal muscle, kidney, heart and spleen with TRIzol (Invitrogen, Gaithersburg, MD, USA). RT–PCR was performed with a primer pair for the secretin receptor 5’-TCGGATGGGGTTCTCCAGC-3’ (sense) and 5’-CTGCACCTCACCATTGAGGAAG-3’ (antisense) which amplifies a cDNA fragment of 402 bp.
Neuropathological examination and X-Gal staining
Dissected adult brains from three age-matched mutants and three control mice were fixed and stained with hematoxylin–eosin and several antibodies, including anti-GFAP (Dako Cytomation Carpinteria, CA, USA), anti-MAP2 (Sigma, St Louis, MO, USA), anti-tryptophan hydroxylase (Sigma) and anti-tyrosine hydroxylase (Chemicon, Temecula, CA, USA). All conditions including tissue fixation, sample preparation and immunocytochemical reactions were the same. The slides were interpreted with animals’ genotype blinded. To determine the expression of the targeted lacZ reporter, dissected adult brains were fixed in 2% glutaraldehyde, 2% formalin, 5 mm EGTA and 2 mm MgCl2 in 0.1 M phosphate buffer (pH 7.3) at room temperature for 30 min. The brains were washed three times with a wash buffer containing 0.1% sodium deoxycholate, 0.2% NP-40 and 2 mm MgCl2 in 0.1 M phosphate buffer for 30 min at room temperature. The sections were then stained with 1 mg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside), 5 mm K4Fe(CN)6 and 5 mm K3Fe(CN)6 in wash buffer overnight at room temperature to visualize β-galactosidase activity. The organs that were stained with X-Gal were embedded in paraffin. Sections were then cut and counterstained with nuclear fast red.
Hippocampal slice preparation and CA1 electrophysiology
Hippocampal slice preparation and electrophysiology were performed as previously described (54). Briefly, hippocampal slices (400 µm) were allowed to equilibrate in a 50% cutting saline (110 mm sucrose, 60 mm NaCl, 3 mm KCl, 1.25 mm NaH2PO4, 28 mm NaHCO3, 0.5 mm CaCl2, 5 mmd-glucose and 0.6 mm ascorbate), 50% ACSF solution (125 mm NaCl, 2.5 mm KCl, 1.24 mm NaH2PO4, 25 mm NaHCO3, 10 mmd-glucose, 2 mm CaCl2 and 1 mm MgCl2) at room temperature for a minimum of 30 min. Extracellular field recordings were obtained from the area CA1 stratum radiatum. Stimulation was supplied with a bipolar teflon-coated, platinum electrode, and recording was obtained with the use of a glass microelectrode filled with ACSF (resistance 1–4 MΩ). Tetani used to evoke CA1 LTP consisted of one set of stimuli. Each set consisted of two trains of 100 Hz frequency stimulation for 1 s, with each train separated by a 20 s interval. Stimulus intensities were adjusted to give pEPSPs with slopes that were ≤50% that of maximum determined from an input/output curve. The 50% maximum stimulus intensity was used for all LTP experiments. Experimental results were used from those slices that exhibited stable baseline synaptic transmission for a minimum of 30 min before the LTP-inducing stimulus was given. Slices from mutant and wild-type animals were used in side-by-side experiments in order to reduce the variability of day-to-day changes in recordings. Data from PPF and LTP experiments were analyzed using a two-way ANOVA, with post hoc tests performed using the method of Bonferroni. Mean differences were considered statistically significant if P<0.05.
Sholl analysis and spine density analysis
The fixed hippocampus of three wild-type and three secretin receptor-deficient mice littermates were processed using the modified rapid Golgi technique as described (55). The 10 best impregnated neurons from the CA1 region of each mouse were drawn using a camera lucida. The Sholl analysis of dendritic trees was performed as previously described (56,57). Briefly, the numbers of apical and basilar dendritic branches that intersected successive concentric circles (placed at 20 µm radial increments) for each cell were counted. An average value for each animal was obtained from the 10 cells evaluated. To calculate the density of spines, the number of spines in 100 µm of first order branches of apical dendrites was counted at high power (100× oil immersion objective) at final magnification of 1000×. For each animal spine, counts were made on a 100 µm segment of 10 dendrites. The average spine count for each animal was calculated, and the average spine counts for mutant and wild-type animals were obtained. Data from the Sholl analysis and spine density experiments were analyzed using an unpaired t-test.
Morris water maze test
The Morris water maze test was performed as described (58), using a 1.3 m diameter circular pool filled with water made opaque by the addition of a white non-toxic paint and was monitored by video camera connected to a digital tracking device (HVS Image, UK). The mice were acclimated for their ability to locate the visible platform (14.5 cm in diameter). Then, the mice were tested for a total of 4 days, eight blocks of trials (two blocks of trials per day) for their ability to locate an unmarked platform that was submerged 1.5 cm beneath the surface of the water. Next, the mice were tested for 2 days, four blocks of trials (two blocks of trials per day) for their ability to locate a new platform position that was submerged and moved to the opposite quadrant position. The Morris water maze test data was analyzed using the two-way (genotype×blocks) analysis of variance (ANOVA) with repeated measures.
Tube test of social dominance
In the tube test, pairs of mice were released headfirst at opposite ends of a white plastic tube (3.7 cm inner diameter, 30.5 cm in length) as described (32). The match ended when one of the mice retreated from the tube. The mouse remaining in the tube was designated the winner (score=1) and the retreating mouse was the loser (score=0). Matches were usually won within 2 min; those lasting >5 min were not scored. Each mouse was tested against three mice of the opposite genotype who were not cagemates. Because the scores of each genotype are not independent, χ2 one sample analysis was used to determine significance by comparing the scores of the mutant mice against an outcome expected by chance (i.e. a 50:50 outcome).
Partition test
The partition test was used to estimate behavioral reactivity of mice to the conspecific behind the clear perforated partition that divides the experimental cage (27×16×12.5 cm3) into halves (33,59). Behavior was scored using a hand-held computer (Psion Workabout mx, Psion Teklogix, Erlanger, KY, USA) with the OBSERVER® program (Noldus Information Technologies, Leesburg, VA, USA). Two primary variables are used to characterize the responses in the partition test: first, the amount of time spent near the partition during the first baseline, familiar partner test and secondly, unfamiliar and familiar-back recognition ratios are calculated. The unfamiliar recognition ratio represents the percentage change in time spent near the partition between the unfamiliar and original baseline partner. The familiar-back recognition ratio is the percentage change in time spent near the partition between the original partner when it is returned to the cage after the unfamiliar partner and the original baseline time spent near the partition. Time spent near the partition, unfamiliar recognition ratio and familiar-back recognition ratio data were analyzed by ANOVA.
Locomotor activity and motor skills
Locomotor activity was evaluated as described by McIlwain et al. (60). Total distance, vertical activity and center distance (the distance traveled in the center of the arena) were recorded and analyzed using ANOVA. Speed was calculated as total distance (cm) divided by time spent moving (s). Motor coordination and balance were tested using an accelerating rotarod (UGO Basile Accelerating Rotarod, Comerio, Italy) as previously described (60). The speed of the rotarod accelerated from 4 to 40 r.p.m. over a 5 min period. Mice were given four trials on two consecutive days, with a maximum time of 300 s (5 min) and a 30–60 min intertrial rest interval. Rotarod data were analyzed using a two-way (genotype×trial) ANOVA with repeated measures.
Pavlovian conditioned fear
Performance in a conditioned fear task was analyzed as described (60,61), using the freeze monitor system (San Diego Instruments). The test chamber (26×22×18 cm3 high) was made of clear Plexiglas and was placed inside a sound attenuated chamber (Med Associates, internal dimensions: 56×38×36 cm3). Context and CS test data were analyzed using a one-way ANOVA.
ACKNOWLEDGEMENTS
We would like to thank Michael Mills and Barbara Antalffy for their excellent technical support, Randy Nelson for the use of his water maze equipment, Donna Kusewitt for her assistance with the pathological examination of the kidney, stomach and pancreas and David Beversdorf and Bennet Givens for their critical reading of the manuscript. Part of this research was supported by grants from NCI NIH and Wellcome Trust (A.B.), the Baylor Mental Retardation and Developmental Disabilities Research Center (funded by the NICHD of NIH HD24064), a grant from NIH HD29256 (D.L.N.) and grants from the Ministry of Education, Science and Culture, Japan, and the Ministry of Health, Labor and Welfare, Japan.
Conflict of Interest statement. None declared.

![Impaired synaptic transmission and LTP of hippocampal CA1 slices. (A) Synaptic transmission was determined from pEPSPs of field recordings from the hippocampal CA1 synapses. Loss of the secretin receptor appears to have a deleterious effect on overall synaptic transmission (F[2,16]=4.095, P=0.0366, m1/m1, n=21; wild-type, n=11). (B) There were no significant changes in the short-term synaptic plasticity by testing the paired-pulse facilitation (PPF). (C) Deficiency of hippocampal LTP induction and maintenance phase in secretin receptor m1/m1 was observed (two-way ANOVA: genotype: F[1,481]=83.97, P<0.0001; time: F[25,481]=1.90, P<0.0001, m1/m1, n=13; +/+, n=11).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/hmg/15/21/10.1093/hmg/ddl402/2/m_ddl40204.jpeg?Expires=1716622023&Signature=vYX2ExQm9g0fBz2QhP5mESQiSJQAxtN0esEMq2xPN7kgSQ3F1r5Egt~meB-d8DUDHN7PO7K8Am9CK9ZT7yf1Lbzgqg6L8osb6CMvZLCo26CX15ms8QNQfN90xnDu8c-ENf9cI89HwqUCnktdDB8k~UhQPbJ78HcBlaVyGIBzSKpgaKYsu5loraeP9Orp2JJwinTgnzHnU3j-xymlC0BjUcUTSSZibejj7X5xfraXBgTBDgx0mXt3dZJ4MAFD6yqYae9mTK2SOMe9vMFs72x2rkr-CSUoKP-hUr65IpnB2Ss4dgk2hK0UNJNW1~8D9gRJthwb0clp0IRyylpdu1QBqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Reversal water maze behavior abnormalities in secretin receptor mutant mice. (A) Hidden platform test (latency). Both secretin receptor-deficient mice (m1/m1) and wild-type littermates (+/+) showed significant reductions in latency (search time) over the eight blocks of training. There was no significant difference between m1/m1 and +/+ (P>0.05). (B) Reversal hidden platform test (latency). Secretin receptor-deficient mice (m1/m1) did not show a decrease in latency over the four blocks of the test when compared with the wild-type mice (+/+) (two-way ANOVA: genotype: F[1,43]=8.656, P<0.005; time: F[3,129]=3.828, P<0.02).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/hmg/15/21/10.1093/hmg/ddl402/2/m_ddl40206.jpeg?Expires=1716622023&Signature=viW2h~9aGxBiQmug3pm0OGLdSDAgZkWxc2iwJAz9vY6ntx2DY7PO1W-hMLxr~23FrdSdImzB2iYeLBSsNuZNf7-T3Y3rXFmUdBs-N3BRWHgwrGJUW04U1v677Jd8dM8sUsTAo4D3kYidzs61OWvwfTcpD2eDByUEnNBmX~v~hOWm-tvYZYNSA4REMl4SY~l11r~PelUBgv451W1wGx-xc2Y6vM0AxnI4-T7IQNIGfTiwTz1DYptFeb~xw8firB9vcG31kZZwhqvkljANODEB69jdSdZAGKIr8bHG4KBJvOfZKYyakupGUqoDSjcHmEP9pfe6tZYqPl-O5qasL2FOMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)